| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1392917 | 1501163 | 2012 | 8 صفحه PDF | دانلود رایگان |
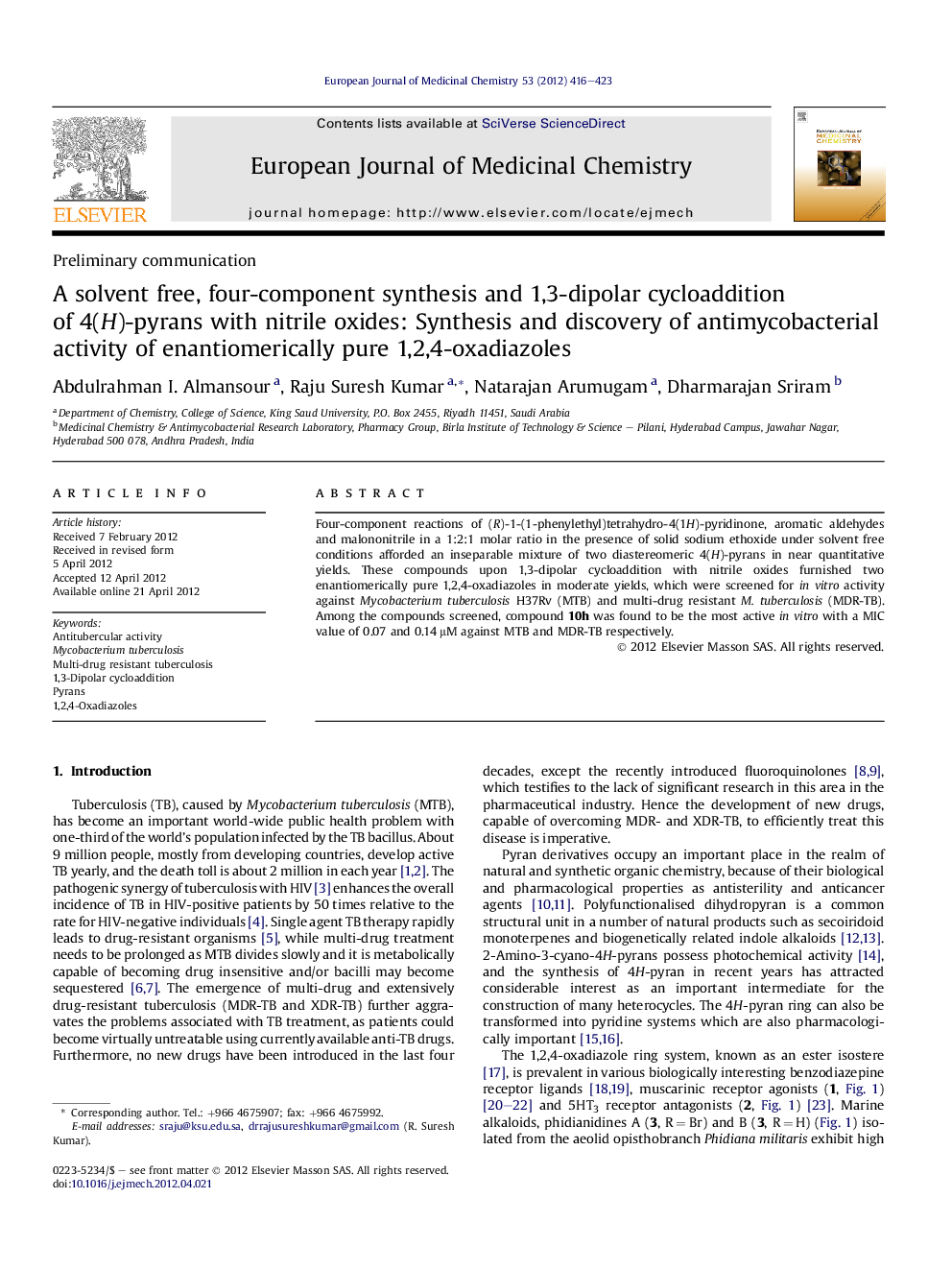
Four-component reactions of (R)-1-(1-phenylethyl)tetrahydro-4(1H)-pyridinone, aromatic aldehydes and malononitrile in a 1:2:1 molar ratio in the presence of solid sodium ethoxide under solvent free conditions afforded an inseparable mixture of two diastereomeric 4(H)-pyrans in near quantitative yields. These compounds upon 1,3-dipolar cycloaddition with nitrile oxides furnished two enantiomerically pure 1,2,4-oxadiazoles in moderate yields, which were screened for in vitro activity against Mycobacterium tuberculosis H37Rv (MTB) and multi-drug resistant M. tuberculosis (MDR-TB). Among the compounds screened, compound 10h was found to be the most active in vitro with a MIC value of 0.07 and 0.14 μM against MTB and MDR-TB respectively.
1,3-Dipolar cycloaddition of 4(H)-pyrans with nitrile oxides furnished two enantiomerically pure 1,2,4-oxadiazoles, these compounds were screened for their in vitro activity against H37Rv (MTB) and (MDR-TB).Figure optionsDownload as PowerPoint slideHighlights
► Diastereomeric 4H-pyrans synthesized in near quantitative yields.
► 1,3-Dipolar cycloaddition of 4H-pyrans with nitrile oxides afforded two enantiomerically pure 1,2,4-oxadiazoles.
► The oxadiazoles displayed excellent in vitro antimycobacterial activity against H37Rv (MTB) and MDR-TB.
Journal: European Journal of Medicinal Chemistry - Volume 53, July 2012, Pages 416–423