| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 144153 | 438922 | 2016 | 6 صفحه PDF | دانلود رایگان |
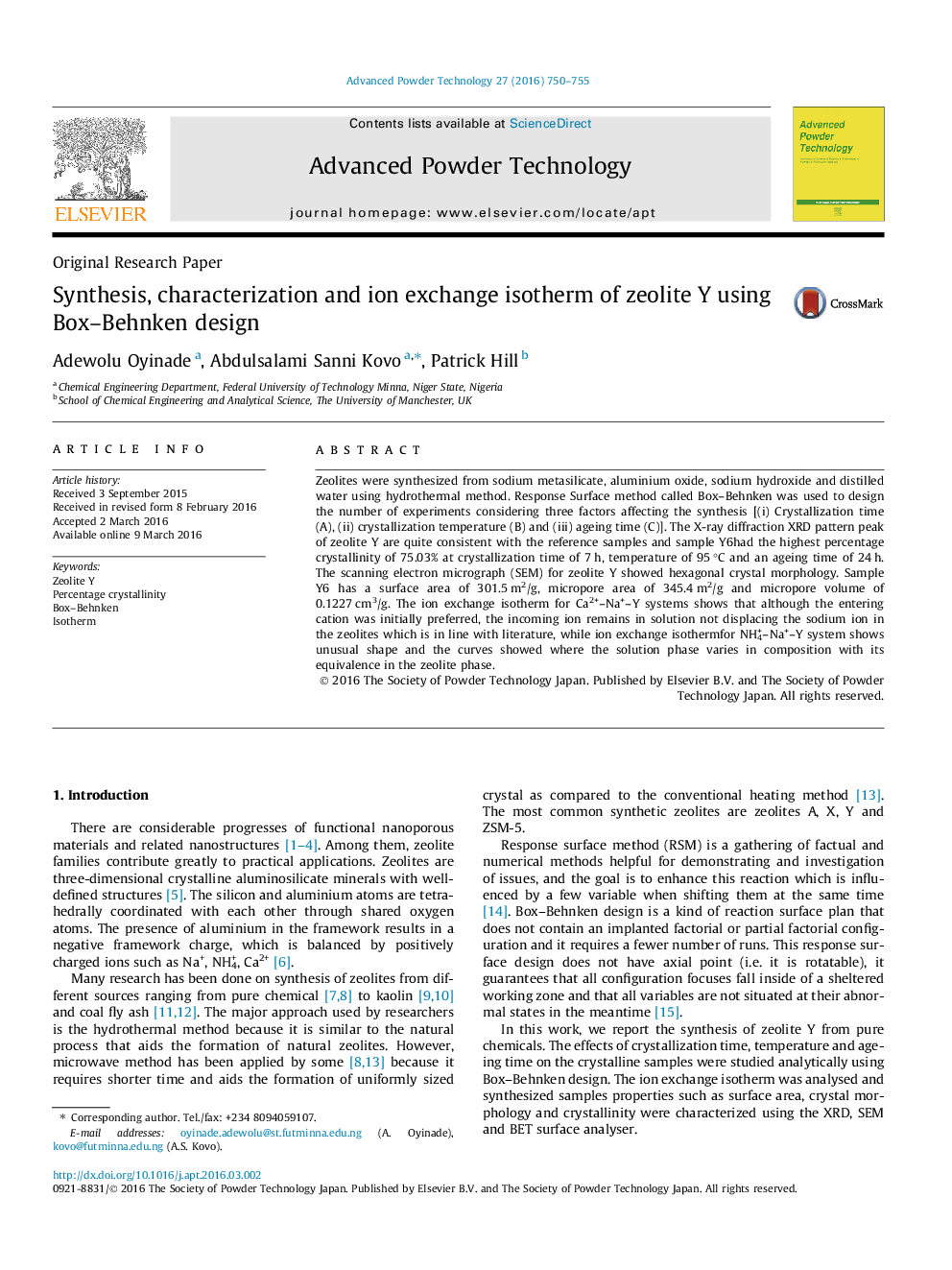
• Low crystallization time and temperature resulted in low crystallinity and sizes.
• Zeolite Y was obtained at best conditions of time and temperature of 7 h, and 95 °C.
• NH4+ exchange zeolite Y showed CEC that increase with crystallization temperature.
• The CEC decrease with increase in time at low crystallization temperature.
Zeolites were synthesized from sodium metasilicate, aluminium oxide, sodium hydroxide and distilled water using hydrothermal method. Response Surface method called Box–Behnken was used to design the number of experiments considering three factors affecting the synthesis [(i) Crystallization time (A), (ii) crystallization temperature (B) and (iii) ageing time (C)]. The X-ray diffraction XRD pattern peak of zeolite Y are quite consistent with the reference samples and sample Y6had the highest percentage crystallinity of 75.03% at crystallization time of 7 h, temperature of 95 °C and an ageing time of 24 h. The scanning electron micrograph (SEM) for zeolite Y showed hexagonal crystal morphology. Sample Y6 has a surface area of 301.5 m2/g, micropore area of 345.4 m2/g and micropore volume of 0.1227 cm3/g. The ion exchange isotherm for Ca2+–Na+–Y systems shows that although the entering cation was initially preferred, the incoming ion remains in solution not displacing the sodium ion in the zeolites which is in line with literature, while ion exchange isothermfor NH4+–Na+–Y system shows unusual shape and the curves showed where the solution phase varies in composition with its equivalence in the zeolite phase.
A 3D plot of crystallization time and temperature representation for zeolites Y percentage crystallinity.Figure optionsDownload as PowerPoint slide
Journal: Advanced Powder Technology - Volume 27, Issue 2, March 2016, Pages 750–755