| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 166131 | 1423442 | 2010 | 5 صفحه PDF | دانلود رایگان |
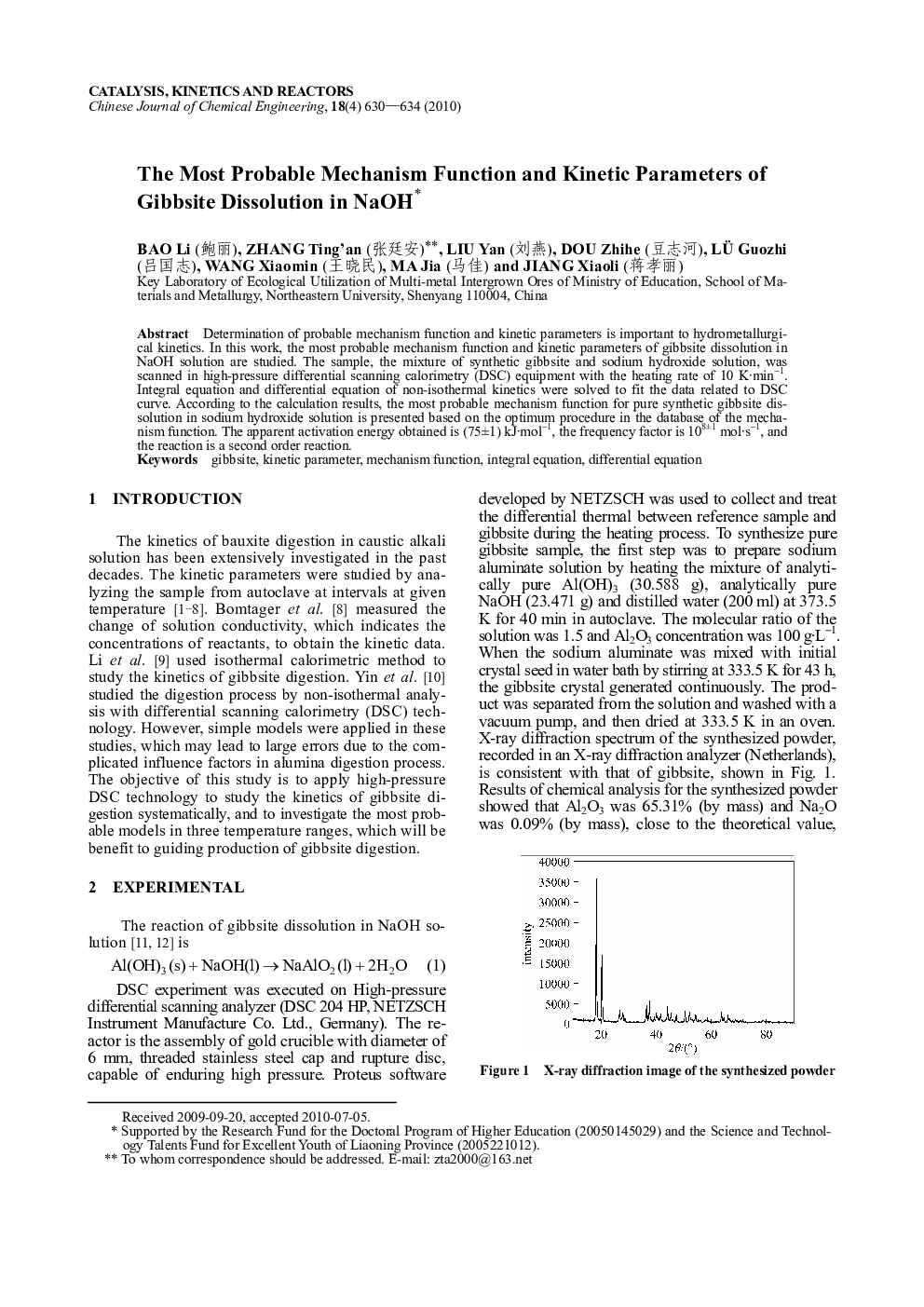
Determination of probable mechanism function and kinetic parameters is important to hydrometallurgical kinetics. In this work, the most probable mechanism function and kinetic parameters of gibbsite dissolution in NaOH solution are studied. The sample, the mixture of synthetic gibbsite and sodium hydroxide solution, was scanned in high-pressure differential scanning calorimetry (DSC) equipment with the heating rate of 10 K·min−1. Integral equation and differential equation of non-isothermal kinetics were solved to fit the data related to DSC curve. According to the calculation results, the most probable mechanism function for pure synthetic gibbsite dissolution in sodium hydroxide solution is presented based on the optimum procedure in the database of the mechanism function. The apparent activation energy obtained is (75±1) kJ·mol−1, the frequency factor is 108±1 mol·s−1, and the reaction is a second order reaction.
Journal: Chinese Journal of Chemical Engineering - Volume 18, Issue 4, August 2010, Pages 630-634