| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 177897 | 459005 | 2007 | 6 صفحه PDF | دانلود رایگان |
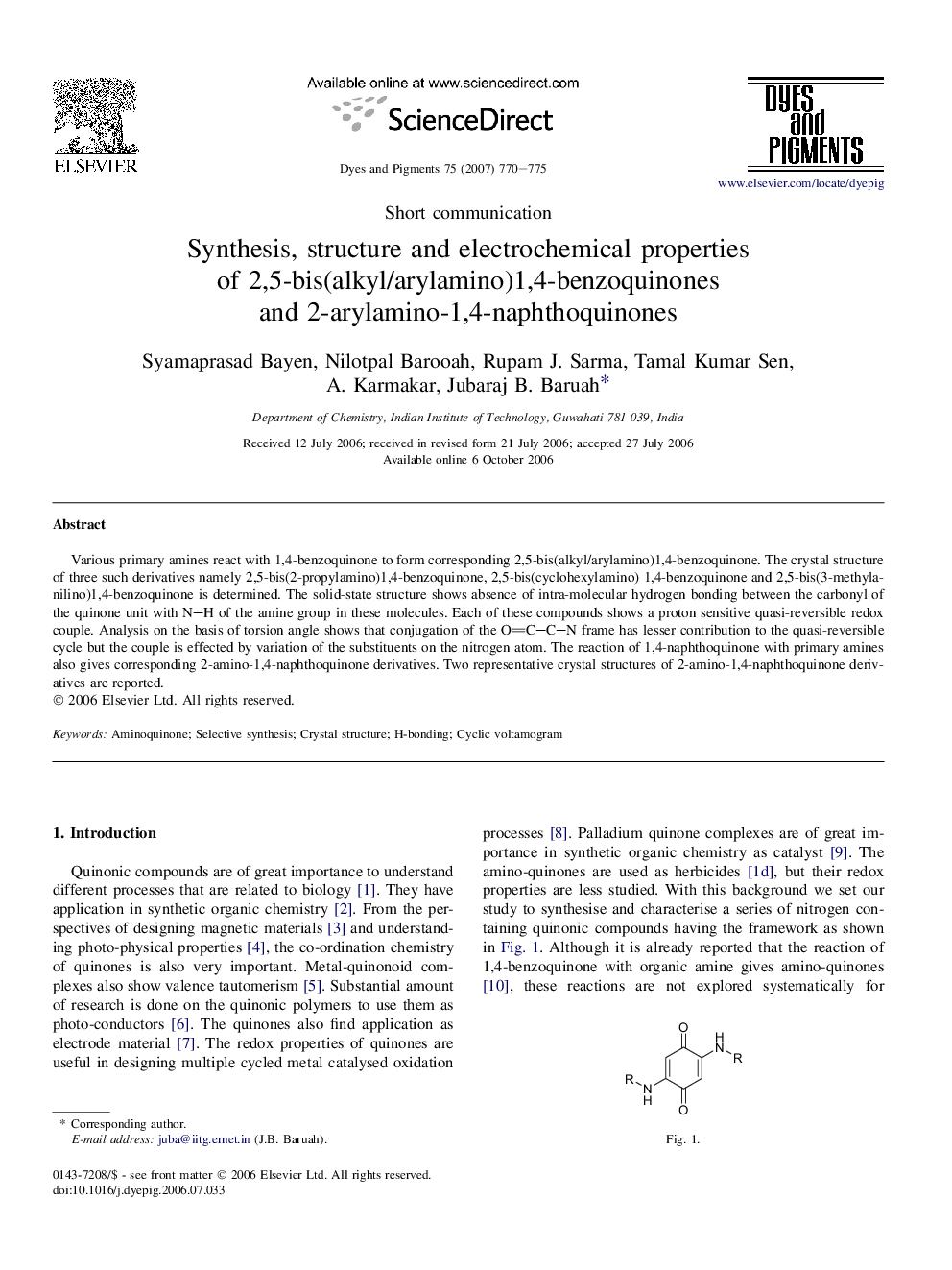
Various primary amines react with 1,4-benzoquinone to form corresponding 2,5-bis(alkyl/arylamino)1,4-benzoquinone. The crystal structure of three such derivatives namely 2,5-bis(2-propylamino)1,4-benzoquinone, 2,5-bis(cyclohexylamino) 1,4-benzoquinone and 2,5-bis(3-methylanilino)1,4-benzoquinone is determined. The solid-state structure shows absence of intra-molecular hydrogen bonding between the carbonyl of the quinone unit with N–H of the amine group in these molecules. Each of these compounds shows a proton sensitive quasi-reversible redox couple. Analysis on the basis of torsion angle shows that conjugation of the OC–C–N frame has lesser contribution to the quasi-reversible cycle but the couple is effected by variation of the substituents on the nitrogen atom. The reaction of 1,4-naphthoquinone with primary amines also gives corresponding 2-amino-1,4-naphthoquinone derivatives. Two representative crystal structures of 2-amino-1,4-naphthoquinone derivatives are reported.
Journal: Dyes and Pigments - Volume 75, Issue 3, 2007, Pages 770–775