| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 2039987 | 1073093 | 2016 | 8 صفحه PDF | دانلود رایگان |
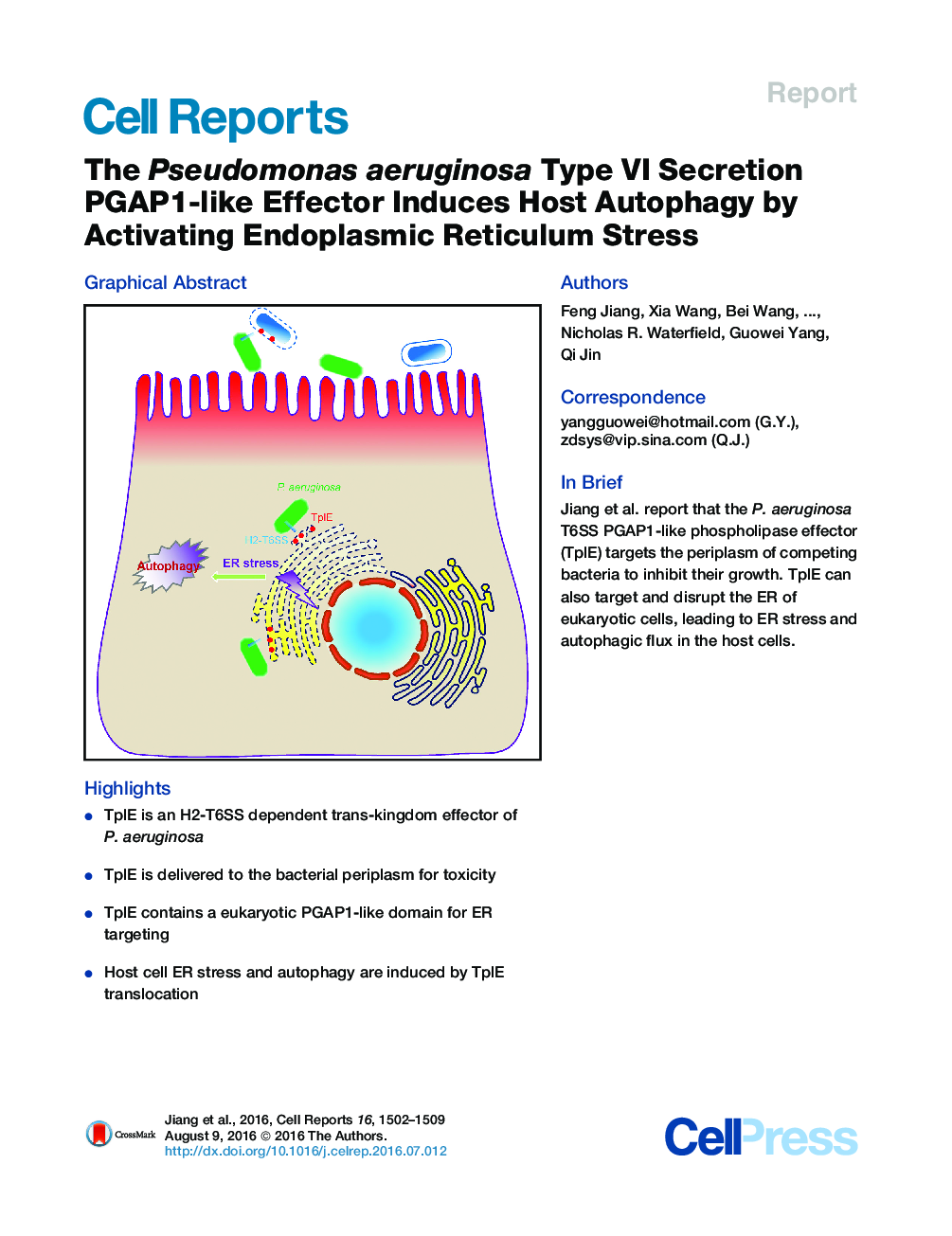
• TplE is an H2-T6SS dependent trans-kingdom effector of P. aeruginosa
• TplE is delivered to the bacterial periplasm for toxicity
• TplE contains a eukaryotic PGAP1-like domain for ER targeting
• Host cell ER stress and autophagy are induced by TplE translocation
SummaryPseudomonas aeruginosa is an opportunistic pathogen that regularly causes nosocomial infections in hospitalized patients. The type VI secretion system (T6SS) is responsible for the secretion of numerous virulence effector proteins that can both interfere with competing microbes and manipulate host cells. Here, we report a detailed investigation of a P. aeruginosa H2-T6SS-dependent phospholipase effector, TplE, which acts as a trans-kingdom toxin. Delivery of TplE to the periplasmic space of rival bacteria leads to growth inhibition. Importantly, TplE, also contains a eukaryotic PGAP1-like domain, which targets the host ER apparatus, ultimately leading to disruption of the ER. TplE activity leads to the activation of the unfolded protein response (UPR) through the IRE1α-XBP1 pathway, enhancing autophagic flux. These findings indicate that this T6SS-delivered phospholipase effector is active against both prokaryotic and eukaryotic cellular targets, highlighting the T6SS as a versatile weapon in the Pseudomonas arsenal.
Graphical AbstractFigure optionsDownload as PowerPoint slide
Journal: - Volume 16, Issue 6, 9 August 2016, Pages 1502–1509