| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 606135 | 1454513 | 2016 | 7 صفحه PDF | دانلود رایگان |
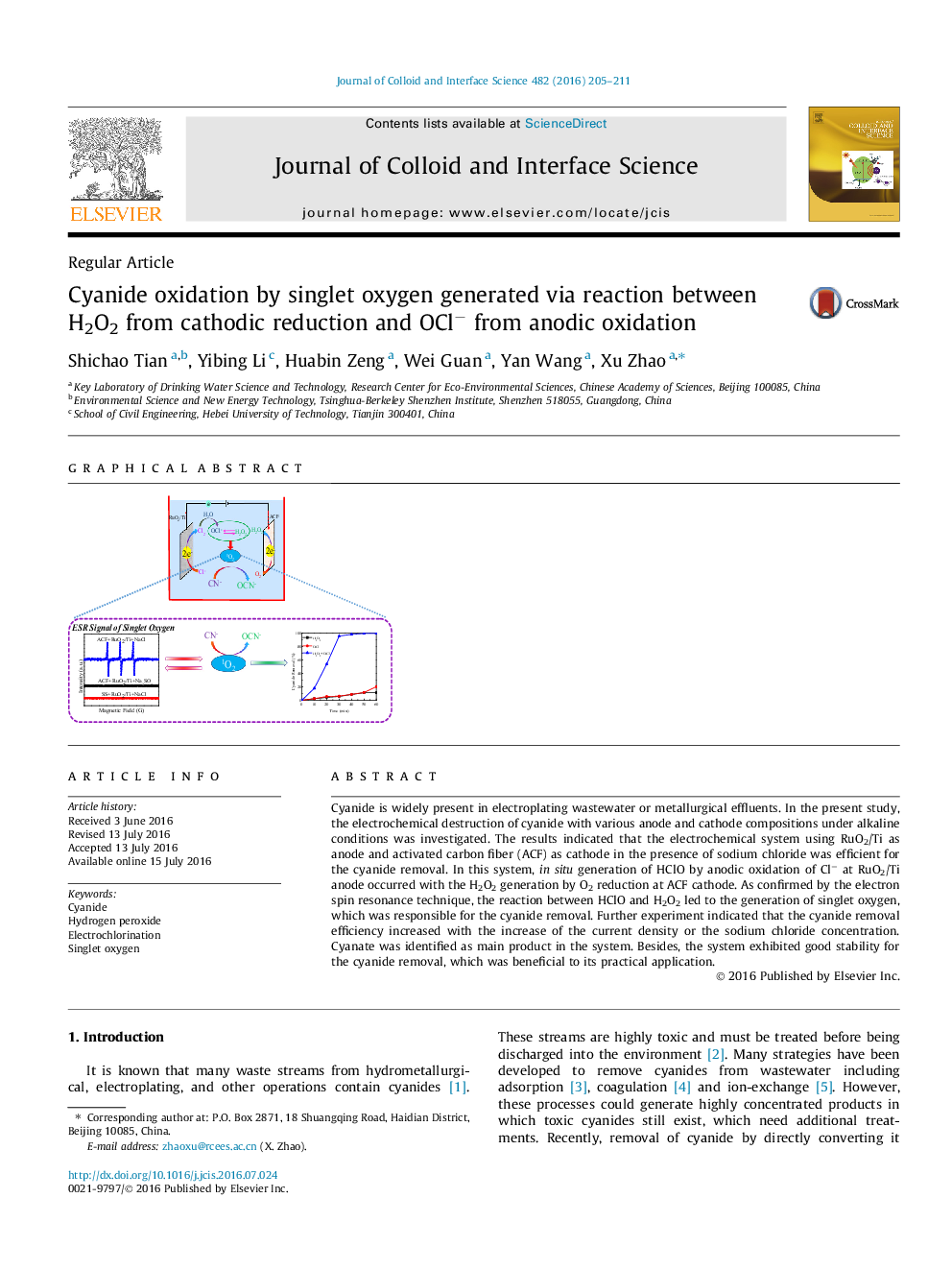
Cyanide is widely present in electroplating wastewater or metallurgical effluents. In the present study, the electrochemical destruction of cyanide with various anode and cathode compositions under alkaline conditions was investigated. The results indicated that the electrochemical system using RuO2/Ti as anode and activated carbon fiber (ACF) as cathode in the presence of sodium chloride was efficient for the cyanide removal. In this system, in situ generation of HClO by anodic oxidation of Cl− at RuO2/Ti anode occurred with the H2O2 generation by O2 reduction at ACF cathode. As confirmed by the electron spin resonance technique, the reaction between HClO and H2O2 led to the generation of singlet oxygen, which was responsible for the cyanide removal. Further experiment indicated that the cyanide removal efficiency increased with the increase of the current density or the sodium chloride concentration. Cyanate was identified as main product in the system. Besides, the system exhibited good stability for the cyanide removal, which was beneficial to its practical application.
Figure optionsDownload high-quality image (154 K)Download as PowerPoint slide
Journal: Journal of Colloid and Interface Science - Volume 482, 15 November 2016, Pages 205–211