| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 608859 | 880610 | 2011 | 8 صفحه PDF | دانلود رایگان |
Dispersions of multi-walled carbon nanotubes (MWNTs) assisted by surfactant adsorption were prepared for a number of ionic and non-ionic surfactants including sodium 4-dodecylbenzenesulfonate (NaDDBS), hexadecyl(trimethyl)azanium bromide (CTAB), sodium dodecane-1-sulfonate (SDS), Pluronic® F68, Pluronic® F127, and Triton® X-100 to examine the effects of nanotube diameter, surfactant concentration, and pH on nanotube dispersability. Nanotube diameter was found to be an important role in surfactant adsorption rendering single-walled carbon nanotube studies as unreliable in predicting MWNT dispersive behavior. Similar to other reports, increasing surfactant concentrations resulted in a solubility plateau. Quantification of nanotube solubility at these plateaus demonstrated that CTAB is the best surfactant for MWNTs at neutral pH conditions. Deviations from neutral pH demonstrated negligible influence on non-ionic surfactant adsorption. In contrast, both cationic and anionic surfactants were found to be poor dispersing aids for highly acidic solutions while, CTAB remained a good surfactant under strongly basic conditions. These pH dependent results were explained in the context of nanotube surface ionization and Debye length variation.
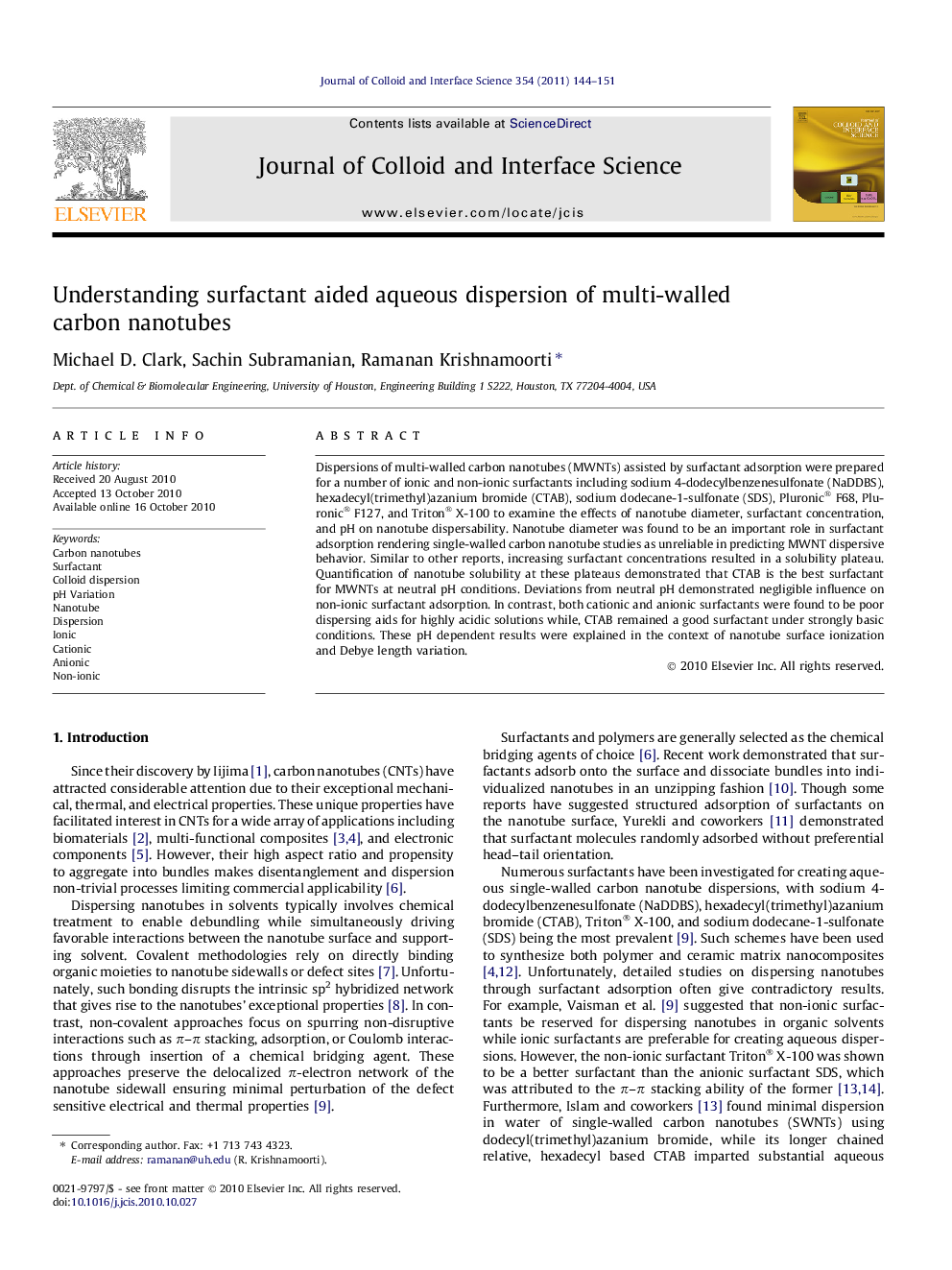
The effect of surfactant concentration, nanotube diameter, and pH on surfactant assisted aqueous carbon nanotube dispersions is presented. Shown is a schematic representation of ionic surfactant adsorption under varying pH.Figure optionsDownload high-quality image (159 K)Download as PowerPoint slideResearch highlights
► Nanotube diameter an important parameter in the efficacy of surfactant assisted dispersion.
► pH has limited role in controlling dispersibility caused by non-ionic surfactants.
► pH dependent dispersibility using ionic surfactants is understood based on surface ionization and Debye length variation.
Journal: Journal of Colloid and Interface Science - Volume 354, Issue 1, 1 February 2011, Pages 144–151