| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 609029 | 880613 | 2010 | 6 صفحه PDF | دانلود رایگان |
Layered double hydroxides (LDHs) weathering in acidic media is one of the main features that affects their applications in drug delivery systems. In this work, the dissolution kinetics of biocompatible Mg–Al LDHs was studied at different initial pH values and solid concentrations using a simple and fast experimental method that coupled flow injection analysis and amperometric detection. A carbonate intercalated sample was used to determine the controlling step of the process and the dissolution mechanism. Finally, the study was extended to an ibuprofen intercalated LDH.The obtained results showed that the weathering process was mainly controlled by the exposed area and surface reactivity of LDHs particles. The dissolution mechanism at the particle surface was described in two steps: fast formation of surface reactive sites by hydroxyl group protonation and slow detachment of metal ions from surface. At strongly acidic conditions, the reaction rate was pH dependent due to the equilibrium between protonated (active) and deprotonated (inactive) hydroxyl groups. On the other hand, at mildly acidic conditions, the dissolution behavior was also ruled by the equilibrium attained between the particle surface reactive sites and the dissolved species. LDHs solubility and dissolution rate presented strong dependence with the interlayer anion. The ibuprofen intercalated sample was more soluble and more rapidly dissolved than the carbonate intercalated one in acetic/acetate buffer. On the other hand, the dissolution mechanism was invariant with the interlayer anion.
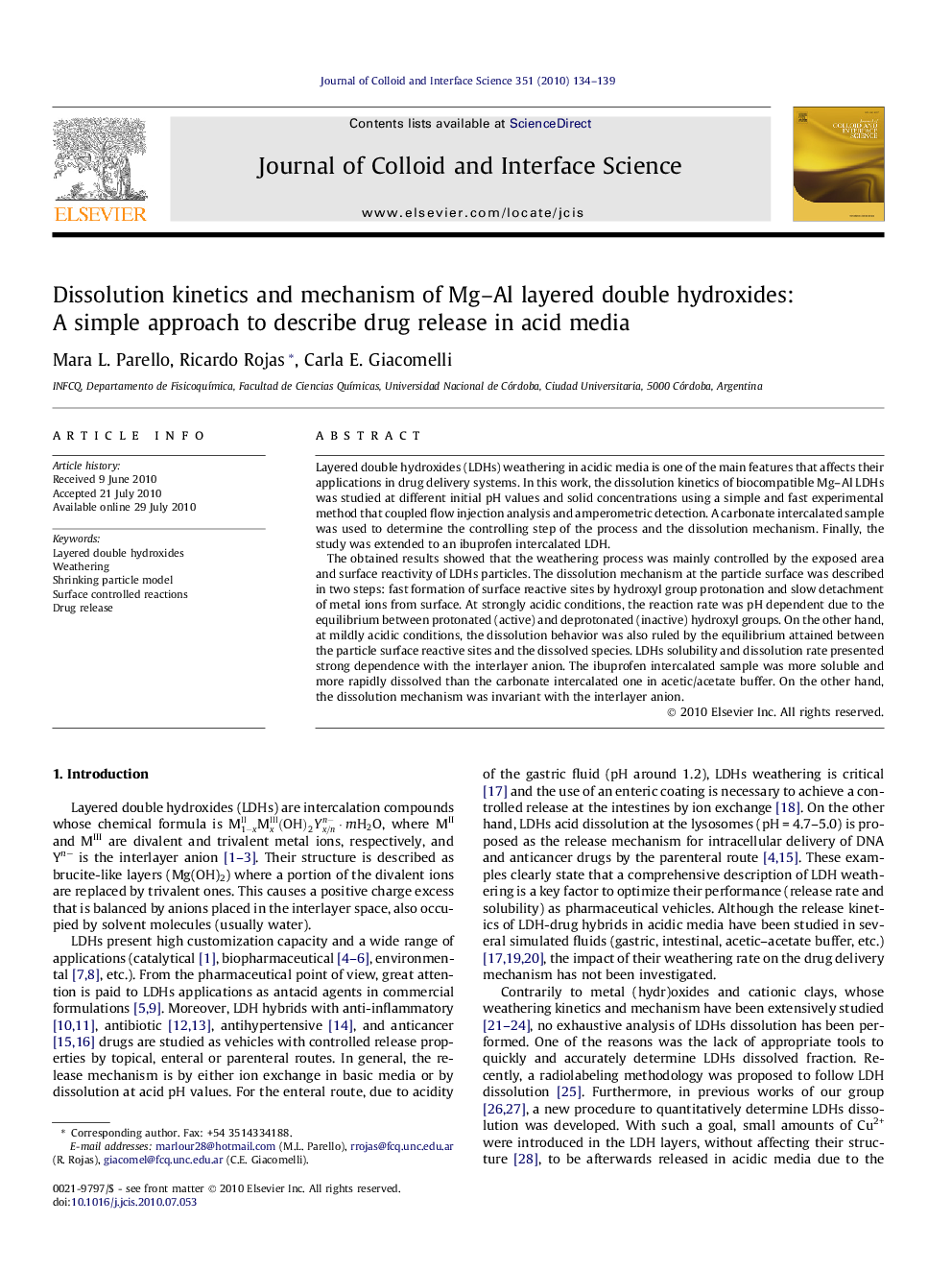
Schematic representation of LDHs particles shrinking in acid medium and the mechanism of the rate-controlling surface reaction.Figure optionsDownload high-quality image (55 K)Download as PowerPoint slideResearch highlights
► LDH weathering kinetics is controlled by a surface reaction with a two step mechanism.
► At high acid concentration, the weathering rate depends on pH and exposed area.
► At low acid concentration, the weathering rate is also ruled by species in solution.
► LDH solubility and weathering rate strongly depend on the interlayer anion.
► The proposed weathering mechanism applies to drug release by LDH in acid media.
Journal: Journal of Colloid and Interface Science - Volume 351, Issue 1, 1 November 2010, Pages 134–139