| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 609170 | 880617 | 2011 | 6 صفحه PDF | دانلود رایگان |
In this paper, the morphology of copper hydroxide nano/microcrystals is finely controlled by adjusting the concentration of surfactant and NH4Cl as well as the reaction temperature, to provide plates, belts, wires, rods and urchin-like spheres, among others. A tree-type multiple head surfactant, bis (amidoethyl-carbamoylethyl) octadecylamine (C18N3), was used to prepare the copper hydroxide nano/microcrystals and it acted as a face-selective additive in the reaction system. The morphology of the Cu(OH)2 microcrystals could be transformed from elliptical plates into truncated square plates as the NH4Cl concentration was increased. The results showed that the Cu(OH)2 crystals were better than the amorphous type of Cu(OH)2 in catalyzing the oxidation of resorcinol with H2O2. Additionally, an investigation of the formation process of the nano/microcrystals was performed, which we believe is valuable for a precise understanding of the formation and fabrication of other metal hydroxides and metal oxides.
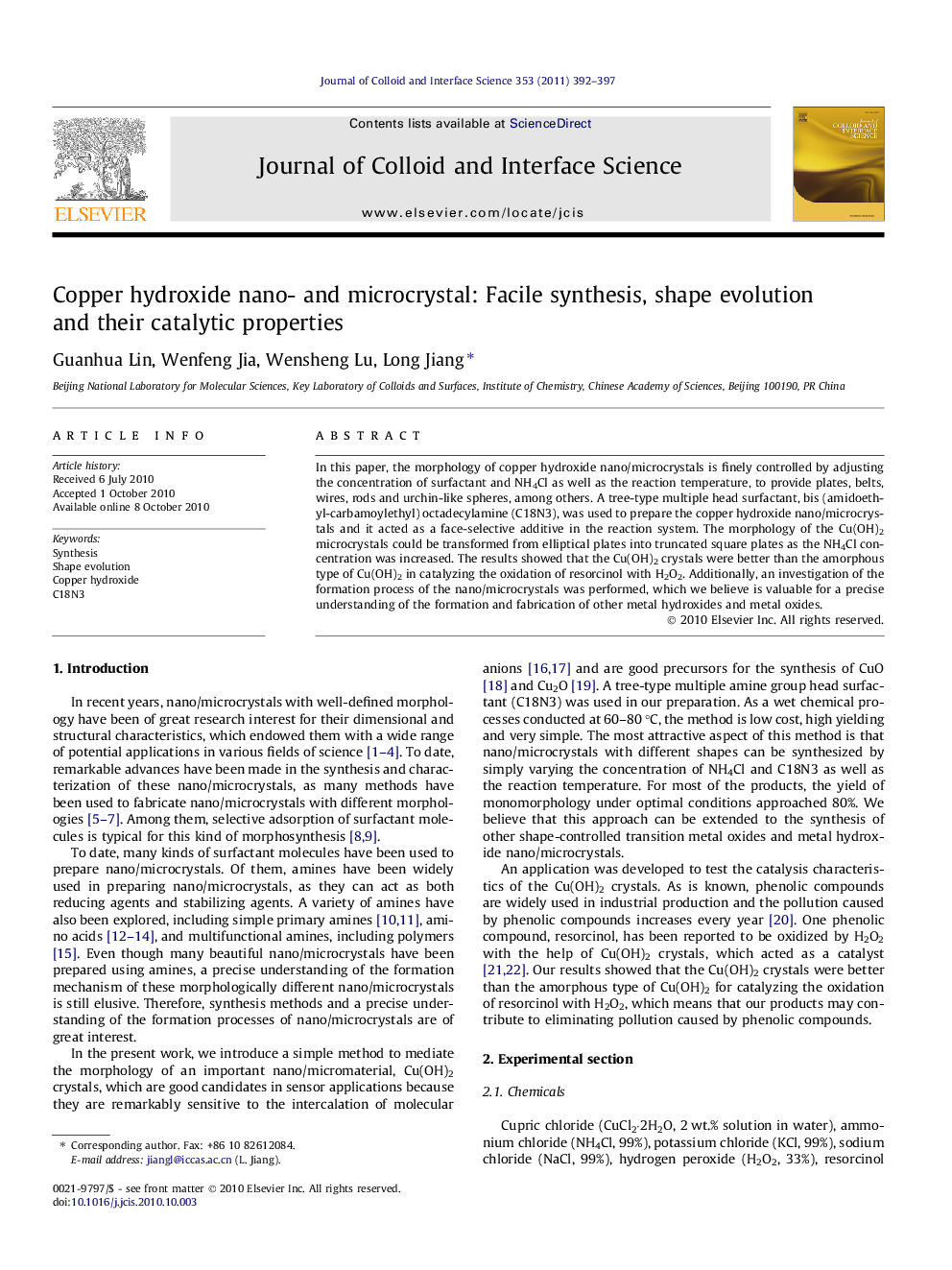
Schematic illustration of the overall formation and shape evolution of Cu(OH)2 nano/microcrystals.Figure optionsDownload high-quality image (69 K)Download as PowerPoint slideResearch highlights
► In this manuscript a simple and useful method had been introduced to fabricate various copper hydroxide nano/microcrystals by just adjusting the concentration of surfactant and NH4Cl as well as reaction temperature, to provide plates, belts, wires, rods and urchin-like spheres among others.
► An application was developed to test the catalysis characteristics of the Cu(OH)2 crystals. Our results showed that the obtained Cu(OH)2 crystals were better than the amorphous type of Cu(OH)2 for catalyzing the oxidation of resorcinol with H2O2, which means that our products may contribute to eliminating pollution caused by phenolic compounds.
► Additionally, an investigation of the formation process of the Cu(OH)2 nano/microcrystals was performed, which we believe is valuable for a precise understanding of the formation and fabrication of other metal hydroxides and metal oxides.
Journal: Journal of Colloid and Interface Science - Volume 353, Issue 2, 15 January 2011, Pages 392–397