| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 610086 | 880639 | 2009 | 6 صفحه PDF | دانلود رایگان |
Gas–solid chromatography was used to determine B2s (gas–solid virial coefficient) values for eight molecular adsorbates interacting with a carbon powder (Carbopack B, Supelco). B2s values were determined by multiple size variant injections within the temperature range of 313–553 K. The molecular adsorbates included: carbon dioxide (CO2); tetrafluoromethane (CF4); hexafluoroethane (C2F6); 1,1-difluoroethane (C2H4F2); 1-chloro-1,1-difluoroethane (C2H3ClF2); dichlorodifluoromethane (CCl2F2); trichlorofluoromethane (CCl3F); and 1,1,1-trichloroethane (C2H3Cl3). Two of these molecules are of special interest because they are “super greenhouse gases”. The global warming potential, GWP, for CF4 is 6500 and for C2F6 is 9200 relative to the reference value of 1 for CO2. The GWP index considers both radiative blocking and molecular lifetime. For these and other industrial greenhouse gases, adsorptive trapping on a carbonaceous solid, which depends on molecule–surface binding energy, could avoid atmospheric release. The temperature variations of the gas–solid virial coefficients in conjunction with van’t Hoff plots were used to find the experimental adsorption energy or binding energy values (E*) for each adsorbate. A molecular mechanics based, rough-surface model was used to calculate the molecule–surface binding energy (Ecal*) using augmented MM2 parameters. The surface model consisted of parallel graphene layers with two separated nanostructures each containing 17 benzene rings arranged in linear strips. The separation of the parallel nanostructures had been optimized in a prior study to appropriately represent molecule–surface interactions for Carbopack B. Linear regressions of E* versus Ecal* for the current data set of eight molecules and the same surface model gave E* = 0.926Ecal* and r2=0.956. A combined set of the current and prior Carbopack B adsorbates studied (linear alkanes, branched alkanes, cyclic alkanes, ethers, and halogenated hydrocarbons) gave a data set with 33 molecules and a regression of E* = 0.991Ecal* and r2=0.968. These results indicated a good correlation between the experimental and the MM2 computed molecule–surface binding energies.
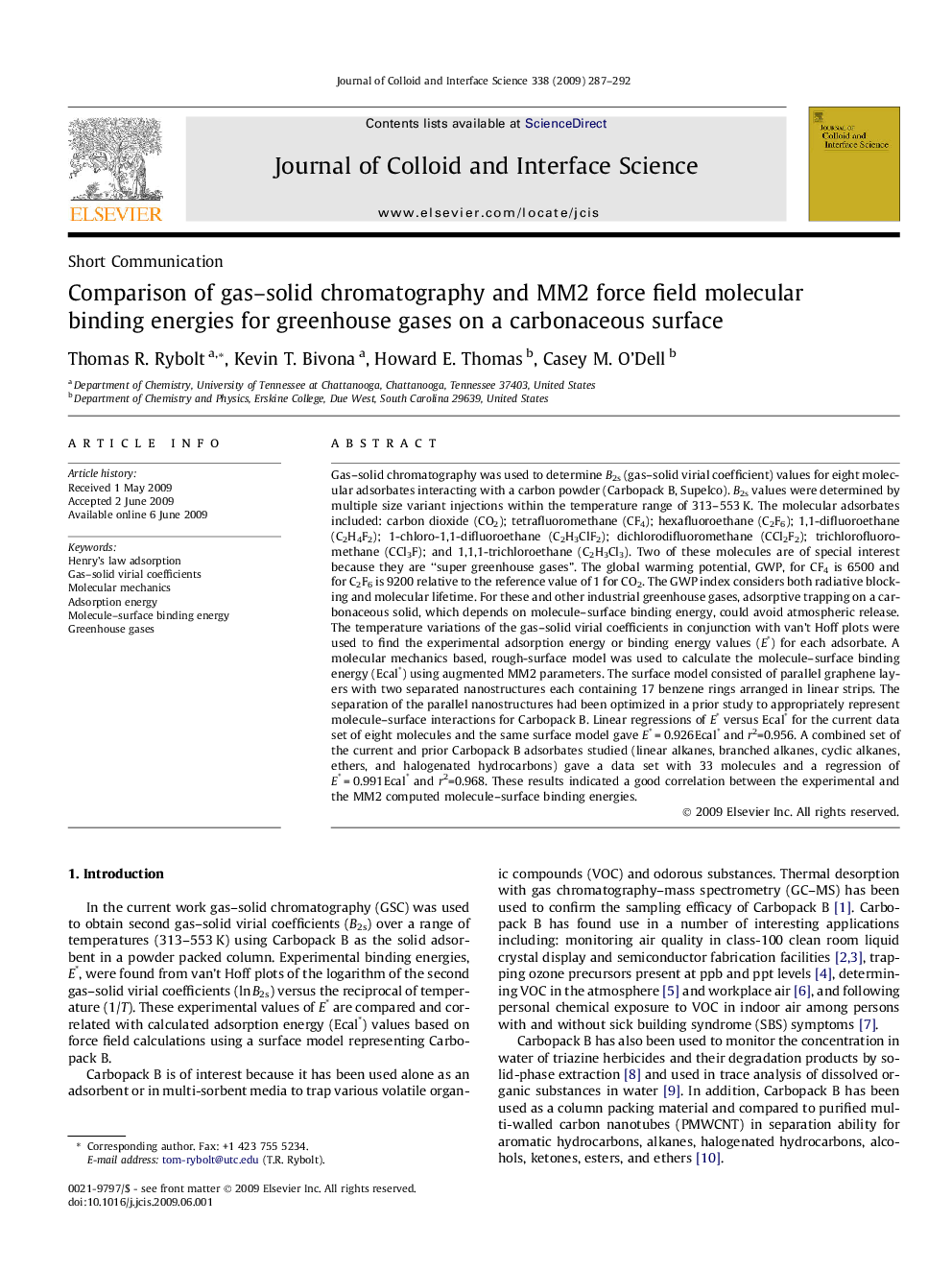
Molecule–surface binding energies were calculated from molecular mechanics (side view of dichlorodifluoromethane on the carbon surface surface model) and compared to experimental values from van’t Hoff plots.Figure optionsDownload high-quality image (48 K)Download as PowerPoint slide
Journal: Journal of Colloid and Interface Science - Volume 338, Issue 1, 1 October 2009, Pages 287–292