| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1304637 | 974840 | 2009 | 4 صفحه PDF | دانلود رایگان |
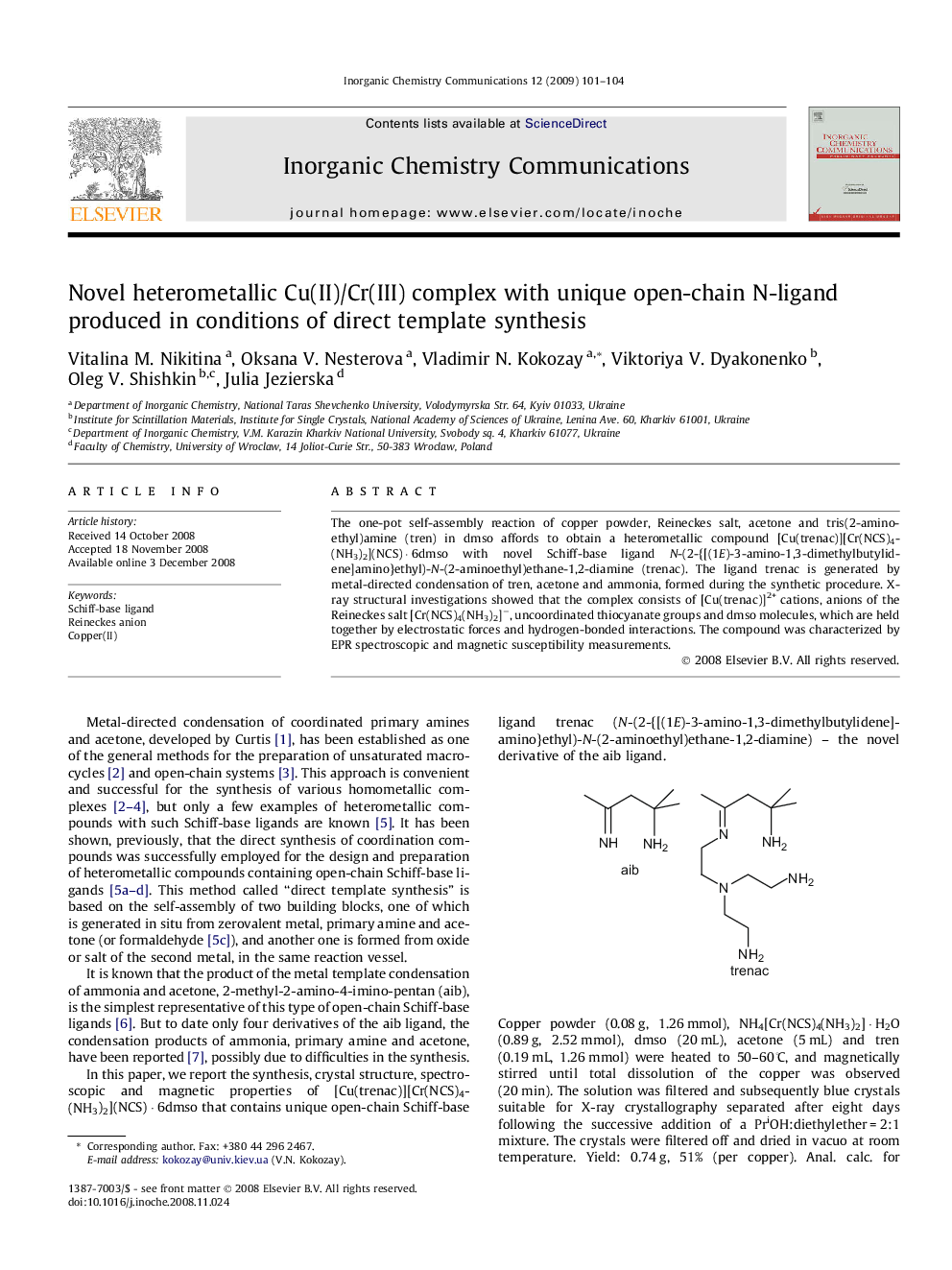
The one-pot self-assembly reaction of copper powder, Reineckes salt, acetone and tris(2-aminoethyl)amine (tren) in dmso affords to obtain a heterometallic compound [Cu(trenac)][Cr(NCS)4(NH3)2](NCS) · 6dmso with novel Schiff-base ligand N-(2-{[(1E)-3-amino-1,3-dimethylbutylidene]amino}ethyl)-N-(2-aminoethyl)ethane-1,2-diamine (trenac). The ligand trenac is generated by metal-directed condensation of tren, acetone and ammonia, formed during the synthetic procedure. X-ray structural investigations showed that the complex consists of [Cu(trenac)]2+ cations, anions of the Reineckes salt [Cr(NCS)4(NH3)2]−, uncoordinated thiocyanate groups and dmso molecules, which are held together by electrostatic forces and hydrogen-bonded interactions. The compound was characterized by EPR spectroscopic and magnetic susceptibility measurements.
New Cu(II)/Cr(III) complex, that contains unique open-chain Schiff-base ligand trenac (N-(2-{[(1E)-3-amino-1,3-dimethylbutylidene]amino}ethyl)-N-(2-aminoethyl)ethane-1,2-diamine), has been prepared using one-pot self-assembly reaction of copper powder, Reineckes salt, tren and acetone in dmso. The structural features, spectroscopic and magnetic properties are reported.Figure optionsDownload as PowerPoint slide
Journal: Inorganic Chemistry Communications - Volume 12, Issue 2, February 2009, Pages 101–104