| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1506482 | 993796 | 2008 | 10 صفحه PDF | دانلود رایگان |
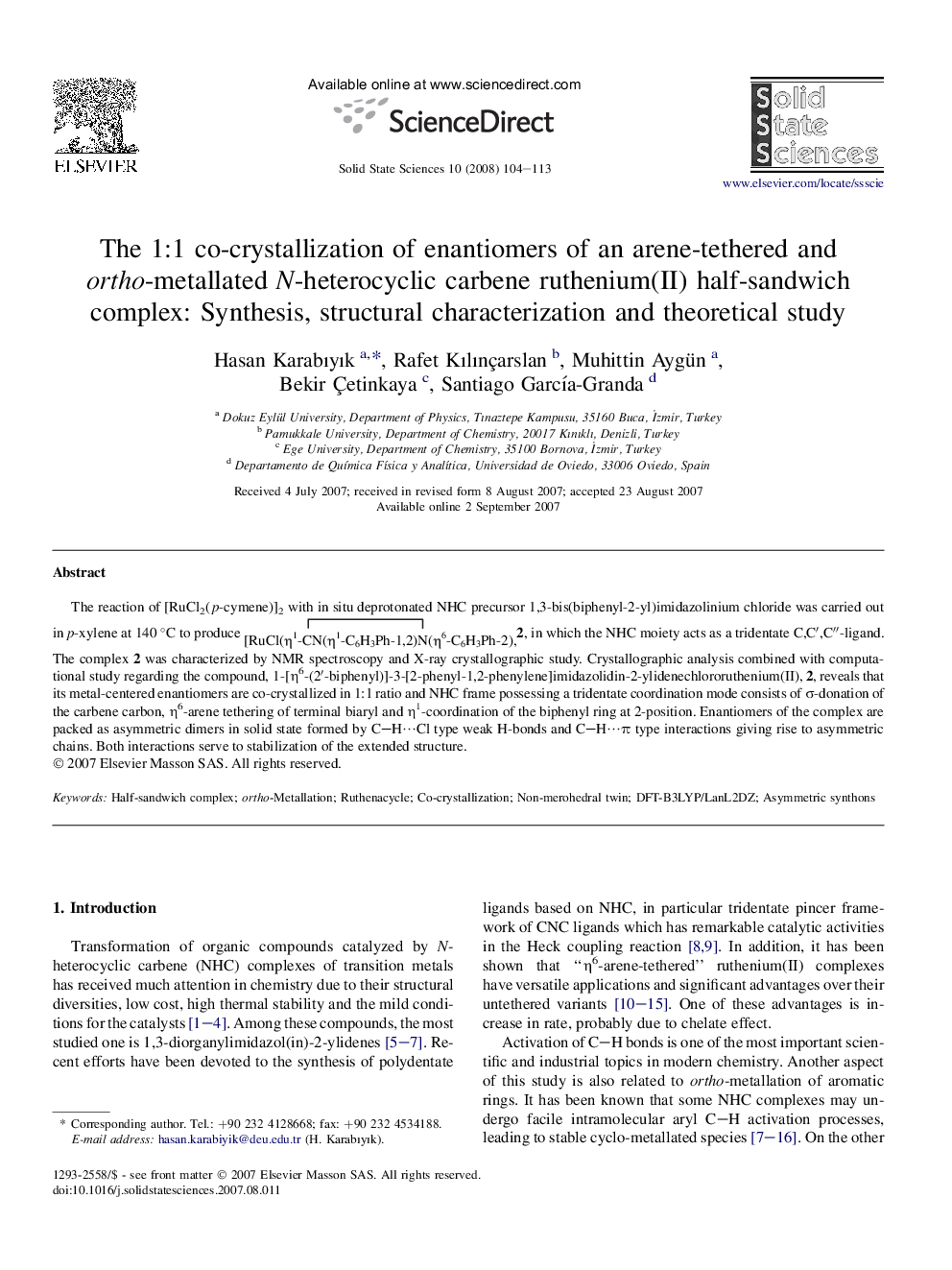
The reaction of [RuCl2(p-cymene)]2 with in situ deprotonated NHC precursor 1,3-bis(biphenyl-2-yl)imidazolinium chloride was carried out in p-xylene at 140 °C to produce 2, in which the NHC moiety acts as a tridentate C,C′,C′′-ligand. The complex 2 was characterized by NMR spectroscopy and X-ray crystallographic study. Crystallographic analysis combined with computational study regarding the compound, 1-[η6-(2′-biphenyl)]-3-[2-phenyl-1,2-phenylene]imidazolidin-2-ylidenechlororuthenium(II), 2, reveals that its metal-centered enantiomers are co-crystallized in 1:1 ratio and NHC frame possessing a tridentate coordination mode consists of σ-donation of the carbene carbon, η6-arene tethering of terminal biaryl and η1-coordination of the biphenyl ring at 2-position. Enantiomers of the complex are packed as asymmetric dimers in solid state formed by C–H⋯Cl type weak H-bonds and C–H⋯π type interactions giving rise to asymmetric chains. Both interactions serve to stabilization of the extended structure.
Figure optionsDownload as PowerPoint slide
Journal: Solid State Sciences - Volume 10, Issue 1, January 2008, Pages 104–113