| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1520639 | 1511786 | 2016 | 5 صفحه PDF | دانلود رایگان |
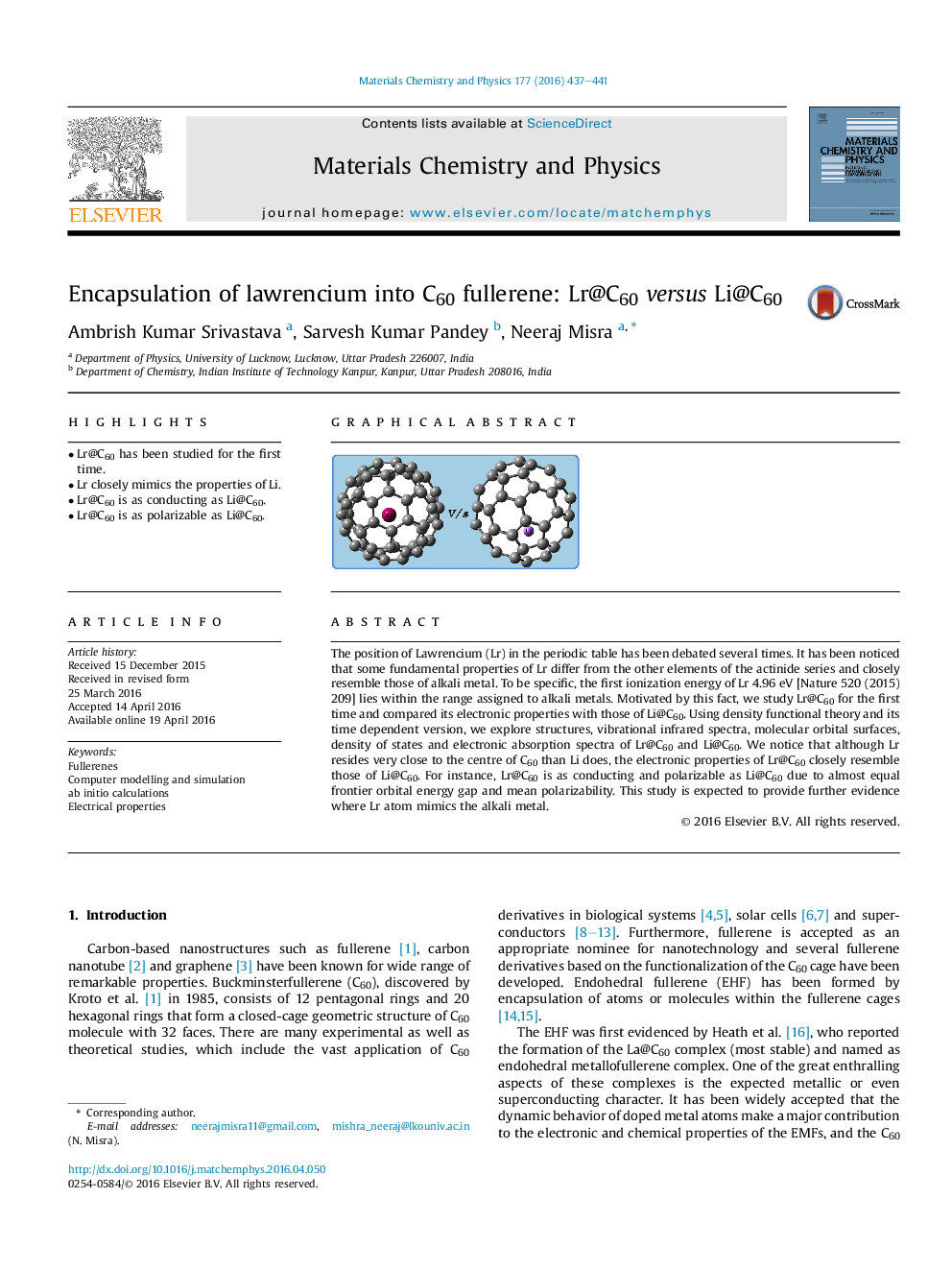
• Lr@C60 has been studied for the first time.
• Lr closely mimics the properties of Li.
• Lr@C60 is as conducting as Li@C60.
• Lr@C60 is as polarizable as Li@C60.
The position of Lawrencium (Lr) in the periodic table has been debated several times. It has been noticed that some fundamental properties of Lr differ from the other elements of the actinide series and closely resemble those of alkali metal. To be specific, the first ionization energy of Lr 4.96 eV [Nature 520 (2015) 209] lies within the range assigned to alkali metals. Motivated by this fact, we study Lr@C60 for the first time and compared its electronic properties with those of Li@C60. Using density functional theory and its time dependent version, we explore structures, vibrational infrared spectra, molecular orbital surfaces, density of states and electronic absorption spectra of Lr@C60 and Li@C60. We notice that although Lr resides very close to the centre of C60 than Li does, the electronic properties of Lr@C60 closely resemble those of Li@C60. For instance, Lr@C60 is as conducting and polarizable as Li@C60 due to almost equal frontier orbital energy gap and mean polarizability. This study is expected to provide further evidence where Lr atom mimics the alkali metal.
Figure optionsDownload as PowerPoint slide
Journal: Materials Chemistry and Physics - Volume 177, 1 July 2016, Pages 437–441