| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1559315 | 999352 | 2007 | 4 صفحه PDF | دانلود رایگان |
عنوان انگلیسی مقاله ISI
Solubility prediction in the ternary systems NaCl–RbCl–H2O, KCl–CsCl–H2O and KBr–CsBr–H2O at 25 ∘C using the ion-interaction model
دانلود مقاله + سفارش ترجمه
دانلود مقاله ISI انگلیسی
رایگان برای ایرانیان
موضوعات مرتبط
مهندسی و علوم پایه
مهندسی مواد
دانش مواد (عمومی)
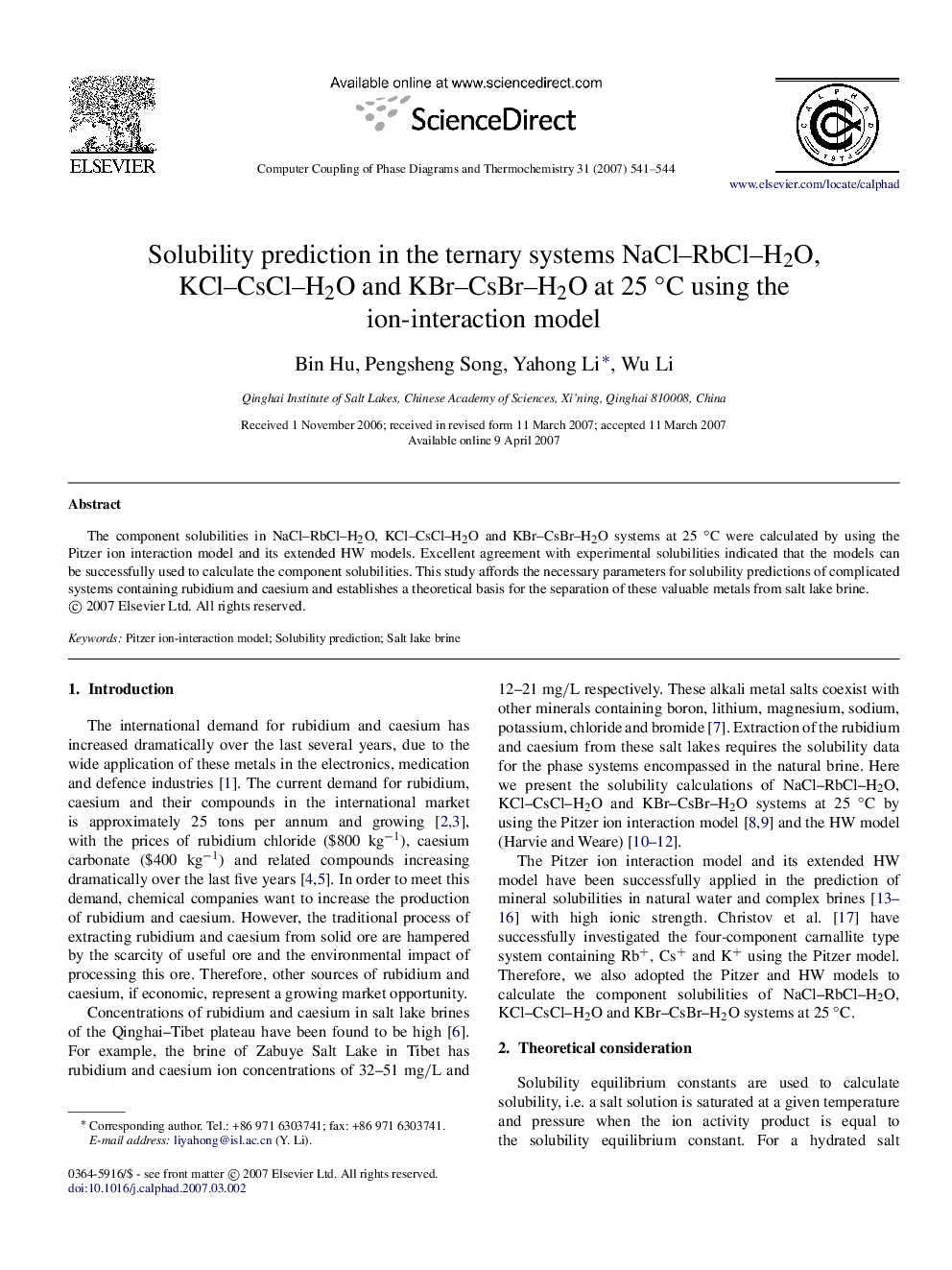
پیش نمایش صفحه اول مقاله
چکیده انگلیسی
The component solubilities in NaCl–RbCl–H2O, KCl–CsCl–H2O and KBr–CsBr–H2O systems at 25 ∘C were calculated by using the Pitzer ion interaction model and its extended HW models. Excellent agreement with experimental solubilities indicated that the models can be successfully used to calculate the component solubilities. This study affords the necessary parameters for solubility predictions of complicated systems containing rubidium and caesium and establishes a theoretical basis for the separation of these valuable metals from salt lake brine.
ناشر
Database: Elsevier - ScienceDirect (ساینس دایرکت)
Journal: Calphad - Volume 31, Issue 4, December 2007, Pages 541–544
Journal: Calphad - Volume 31, Issue 4, December 2007, Pages 541–544
نویسندگان
Bin Hu, Pengsheng Song, Yahong Li, Wu Li,