| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 156385 | 456930 | 2010 | 10 صفحه PDF | دانلود رایگان |
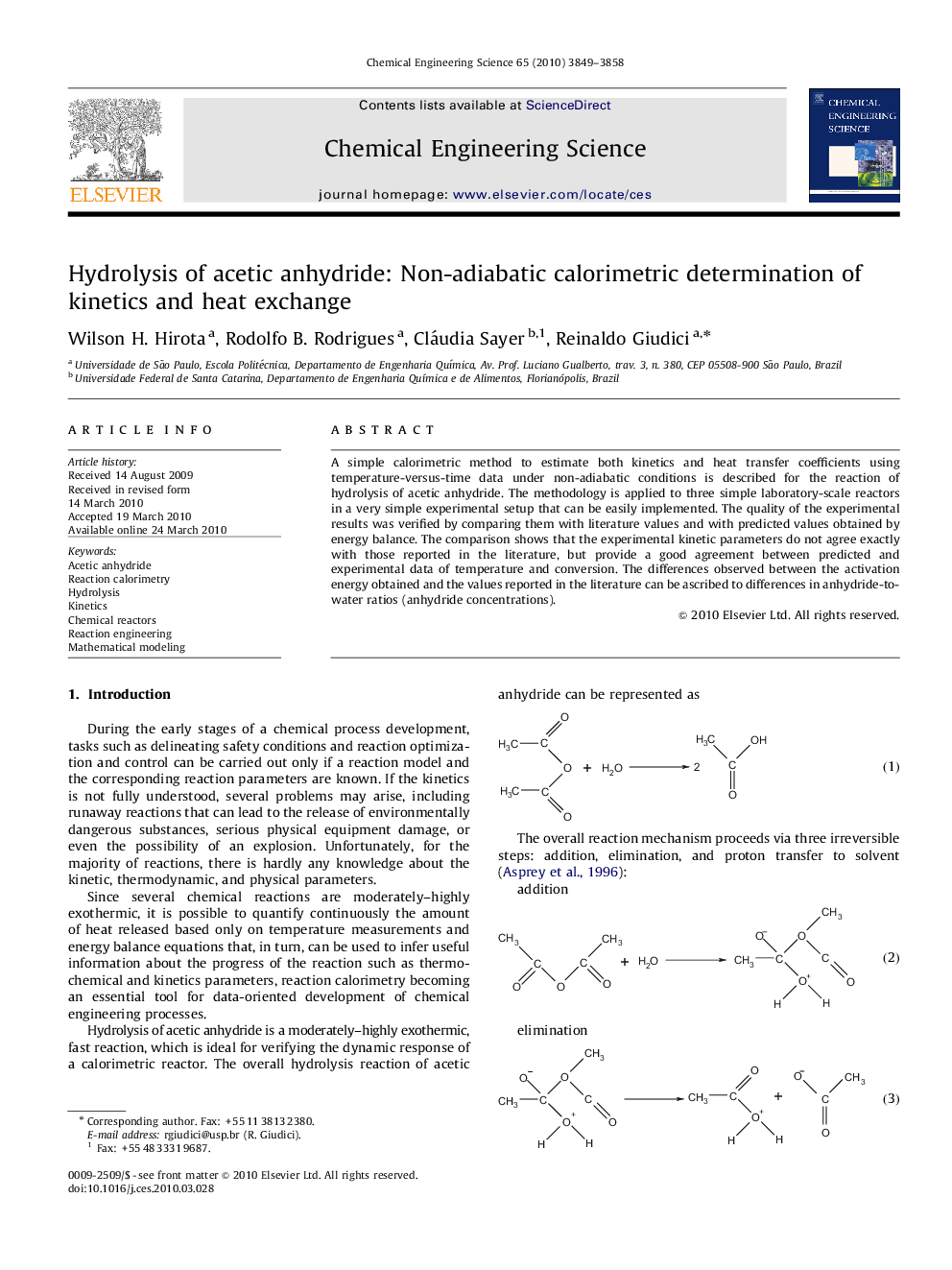
A simple calorimetric method to estimate both kinetics and heat transfer coefficients using temperature-versus-time data under non-adiabatic conditions is described for the reaction of hydrolysis of acetic anhydride. The methodology is applied to three simple laboratory-scale reactors in a very simple experimental setup that can be easily implemented. The quality of the experimental results was verified by comparing them with literature values and with predicted values obtained by energy balance. The comparison shows that the experimental kinetic parameters do not agree exactly with those reported in the literature, but provide a good agreement between predicted and experimental data of temperature and conversion. The differences observed between the activation energy obtained and the values reported in the literature can be ascribed to differences in anhydride-to-water ratios (anhydride concentrations).
Journal: Chemical Engineering Science - Volume 65, Issue 12, 15 June 2010, Pages 3849–3858