| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 2039079 | 1073022 | 2016 | 9 صفحه PDF | دانلود رایگان |
• Astrin co-purifies and localizes with Mid1 and Mid2 to cytokinetic microtubules
• Mid2 ubiquitinates astrin on K409 and targets it for degradation during cytokinesis
• Mid2 depletion leads to astrin stabilization and increased cytokinetic defects
• K409A astrin accumulates at cytokinetic microtubules and leads to cytokinetic defects
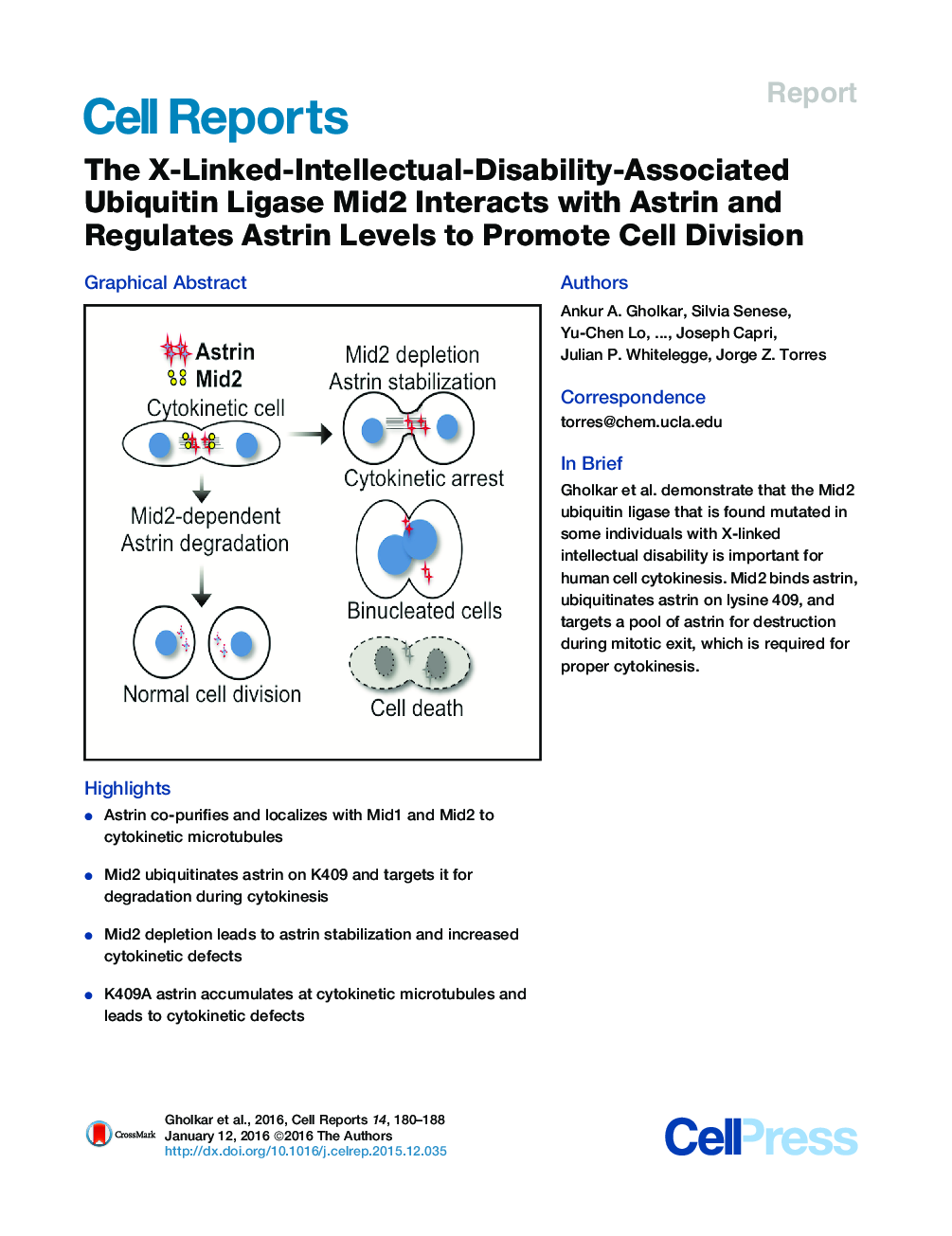
SummaryMid1 and Mid2 are ubiquitin ligases that regulate microtubule dynamics and whose mutation is associated with X-linked developmental disorders. We show that astrin, a microtubule-organizing protein, co-purifies with Mid1 and Mid2, has an overlapping localization with Mid1 and Mid2 at intercellular bridge microtubules, is ubiquitinated by Mid2 on lysine 409, and is degraded during cytokinesis. Mid2 depletion led to astrin stabilization during cytokinesis, cytokinetic defects, multinucleated cells, and cell death. Similarly, expression of a K409A mutant astrin in astrin-depleted cells led to the accumulation of K409A on intercellular bridge microtubules and an increase in cytokinetic defects, multinucleated cells, and cell death. These results indicate that Mid2 regulates cell division through the ubiquitination of astrin on K409, which is critical for its degradation and proper cytokinesis. These results could help explain how mutation of MID2 leads to misregulation of microtubule organization and the downstream disease pathology associated with X-linked intellectual disabilities.
Graphical AbstractFigure optionsDownload as PowerPoint slide
Journal: - Volume 14, Issue 2, 12 January 2016, Pages 180–188