| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 38869 | 45794 | 2016 | 10 صفحه PDF | دانلود رایگان |
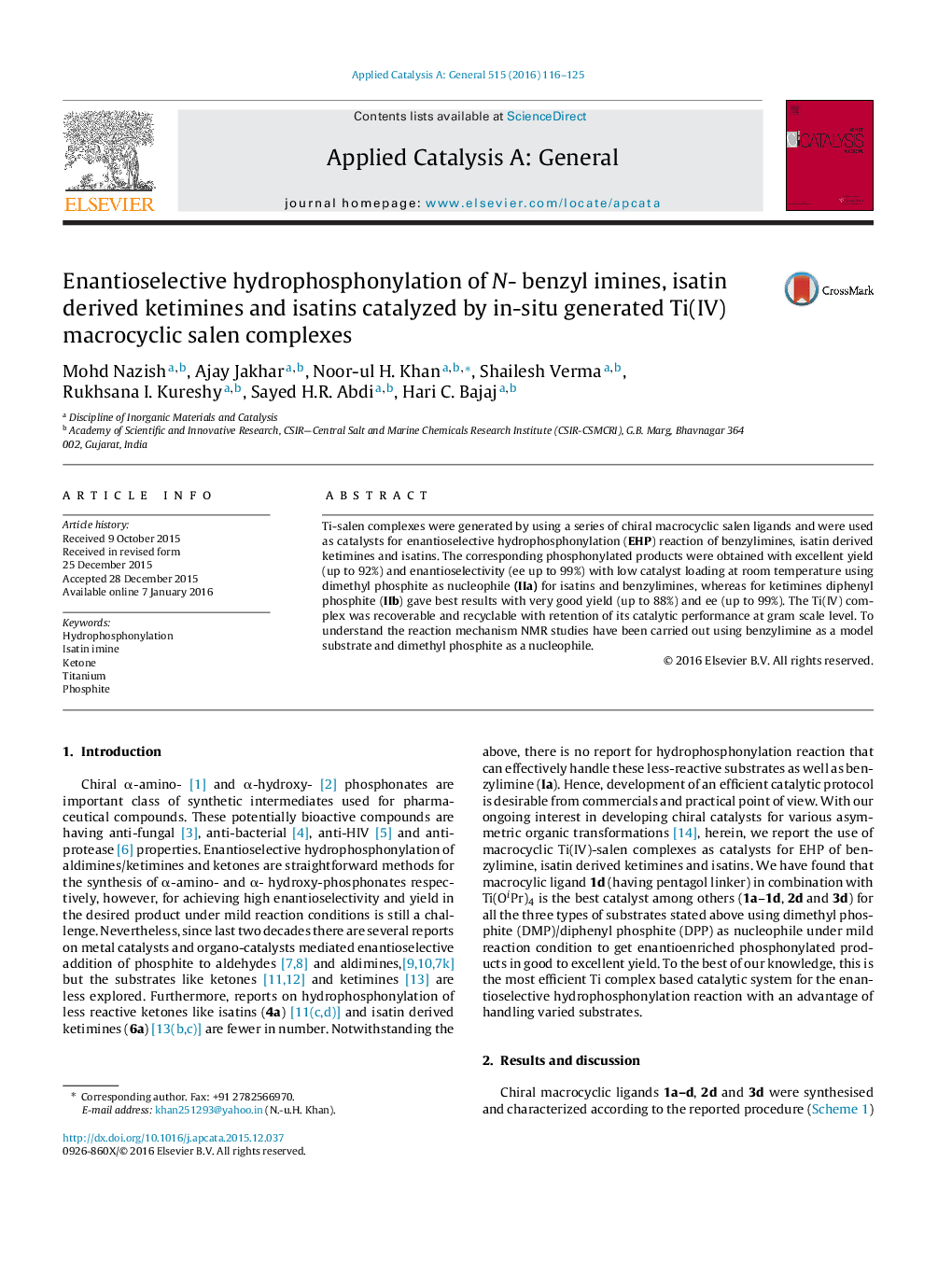
• Macrocyclic salen-titanium complex.
• Catalyst recyclable five times.
• Asymmetric hydrophosphonylation reaction with 21 examples.
• Simple, ideal and efficient catlytic protocol.
• Proposed a mechanism based on NMR spectroscopic study.
Ti-salen complexes were generated by using a series of chiral macrocyclic salen ligands and were used as catalysts for enantioselective hydrophosphonylation (EHP) reaction of benzylimines, isatin derived ketimines and isatins. The corresponding phosphonylated products were obtained with excellent yield (up to 92%) and enantioselectivity (ee up to 99%) with low catalyst loading at room temperature using dimethyl phosphite as nucleophile (IIa) for isatins and benzylimines, whereas for ketimines diphenyl phosphite (IIb) gave best results with very good yield (up to 88%) and ee (up to 99%). The Ti(IV) complex was recoverable and recyclable with retention of its catalytic performance at gram scale level. To understand the reaction mechanism NMR studies have been carried out using benzylimine as a model substrate and dimethyl phosphite as a nucleophile.
Figure optionsDownload high-quality image (154 K)Download as PowerPoint slide
Journal: Applied Catalysis A: General - Volume 515, 10 April 2016, Pages 116–125