| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 443219 | 692690 | 2016 | 11 صفحه PDF | دانلود رایگان |
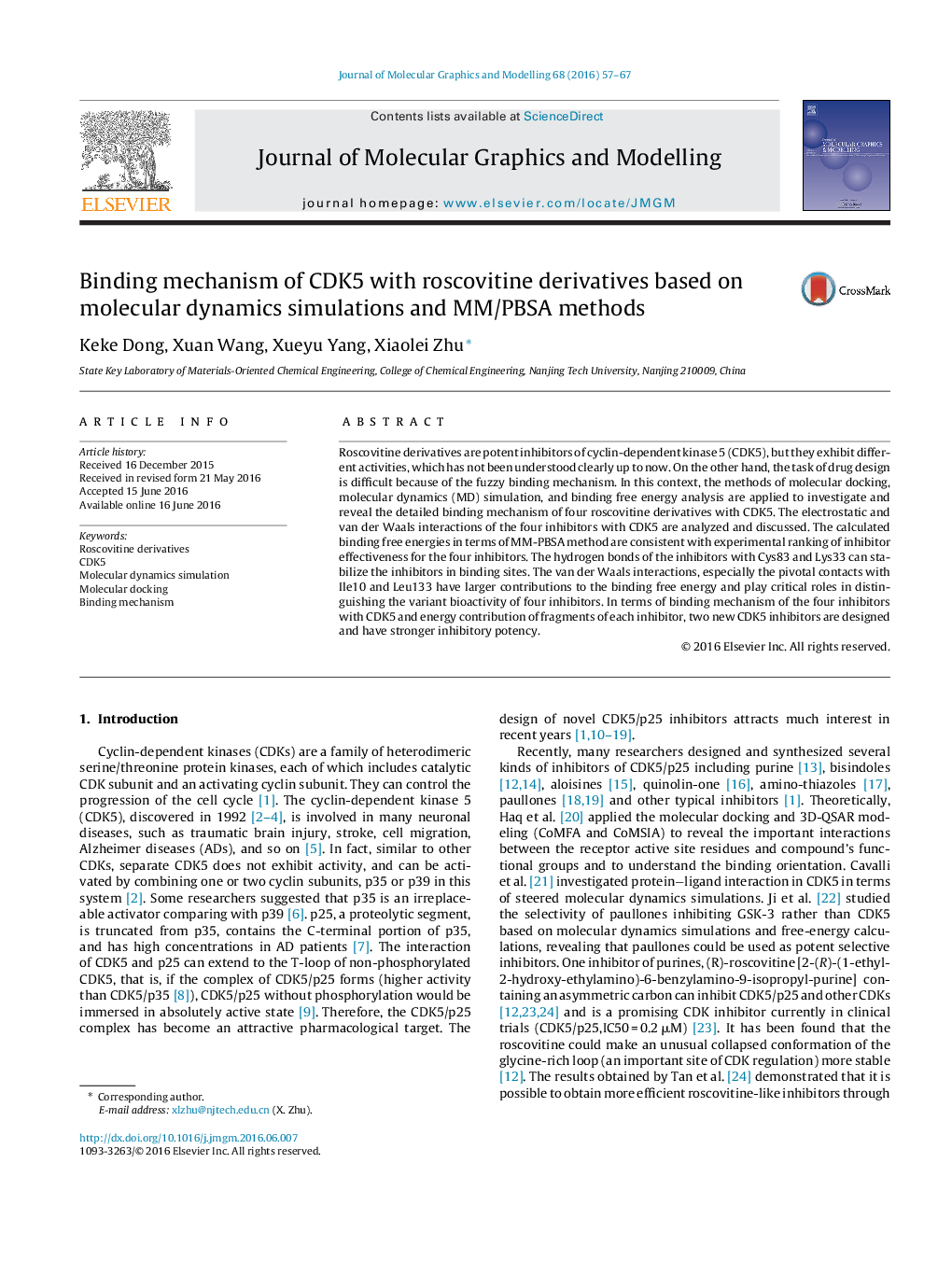
• The computed ΔGbind of CDK5/inhibitor are qualitatively consistent with experimental IC50 results.
• The stable hydrogen bonds with Cys83 and Lys33 are favorable to the binding affinity of four CDK5/inhibitor complexes.
• The stronger van der Waals interactions play an important role in distinguishing the binding affinity of the four inhibitors.
• The binding mechanism of roscovitine derivative inhibitors with CDK5 is revealed.
• The two newly designed inhibitors have stronger inhibitory potency to CDK5 than A1.
Roscovitine derivatives are potent inhibitors of cyclin-dependent kinase 5 (CDK5), but they exhibit different activities, which has not been understood clearly up to now. On the other hand, the task of drug design is difficult because of the fuzzy binding mechanism. In this context, the methods of molecular docking, molecular dynamics (MD) simulation, and binding free energy analysis are applied to investigate and reveal the detailed binding mechanism of four roscovitine derivatives with CDK5. The electrostatic and van der Waals interactions of the four inhibitors with CDK5 are analyzed and discussed. The calculated binding free energies in terms of MM-PBSA method are consistent with experimental ranking of inhibitor effectiveness for the four inhibitors. The hydrogen bonds of the inhibitors with Cys83 and Lys33 can stabilize the inhibitors in binding sites. The van der Waals interactions, especially the pivotal contacts with Ile10 and Leu133 have larger contributions to the binding free energy and play critical roles in distinguishing the variant bioactivity of four inhibitors. In terms of binding mechanism of the four inhibitors with CDK5 and energy contribution of fragments of each inhibitor, two new CDK5 inhibitors are designed and have stronger inhibitory potency.
Figure optionsDownload high-quality image (225 K)Download as PowerPoint slideThe molecular dynamics simulation was utilized to reveal the binding mechanism of the four Roscovitine derivative inhibitors with CDK5. Based on the above results and thermodynamic analysis, and new inhibitors are designed and predicted to have relatively higher bioactivity.
Journal: Journal of Molecular Graphics and Modelling - Volume 68, July 2016, Pages 57–67