| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 55202 | 47043 | 2012 | 5 صفحه PDF | دانلود رایگان |
Protected mesoporous SBA-15 phase was synthesized by grafting the complex Chloro (S,S)(−)[N-3-tert-butyl-5-chloromethyl salicylidene]-N′-[3′,5′-di tert-butyl salicylidene] 1,1′-binapthyl-2,2′-diamine manganese(III) through the reactive 3-aminopropyl trimethoxysilane(3-APTMS) group. The surface properties of the functionalized catalyst were analyzed by a series of characterization techniques like elemental analysis, XRD, N2 sorption measurement isotherm, FT-IR, XPS, and solid state 13C NMR. The screening of the catalyst Mn(III)-L-SBA-15 and neat Mn(III)-L complexes was done for the oxidation reaction of thioanisole (methyl phenyl sulfide) using TBHP as an oxidant. Mn(III)-L-SBA-15 catalyst shows higher activities and exhibit enantiomeric excess comparable to homogeneous catalyst.
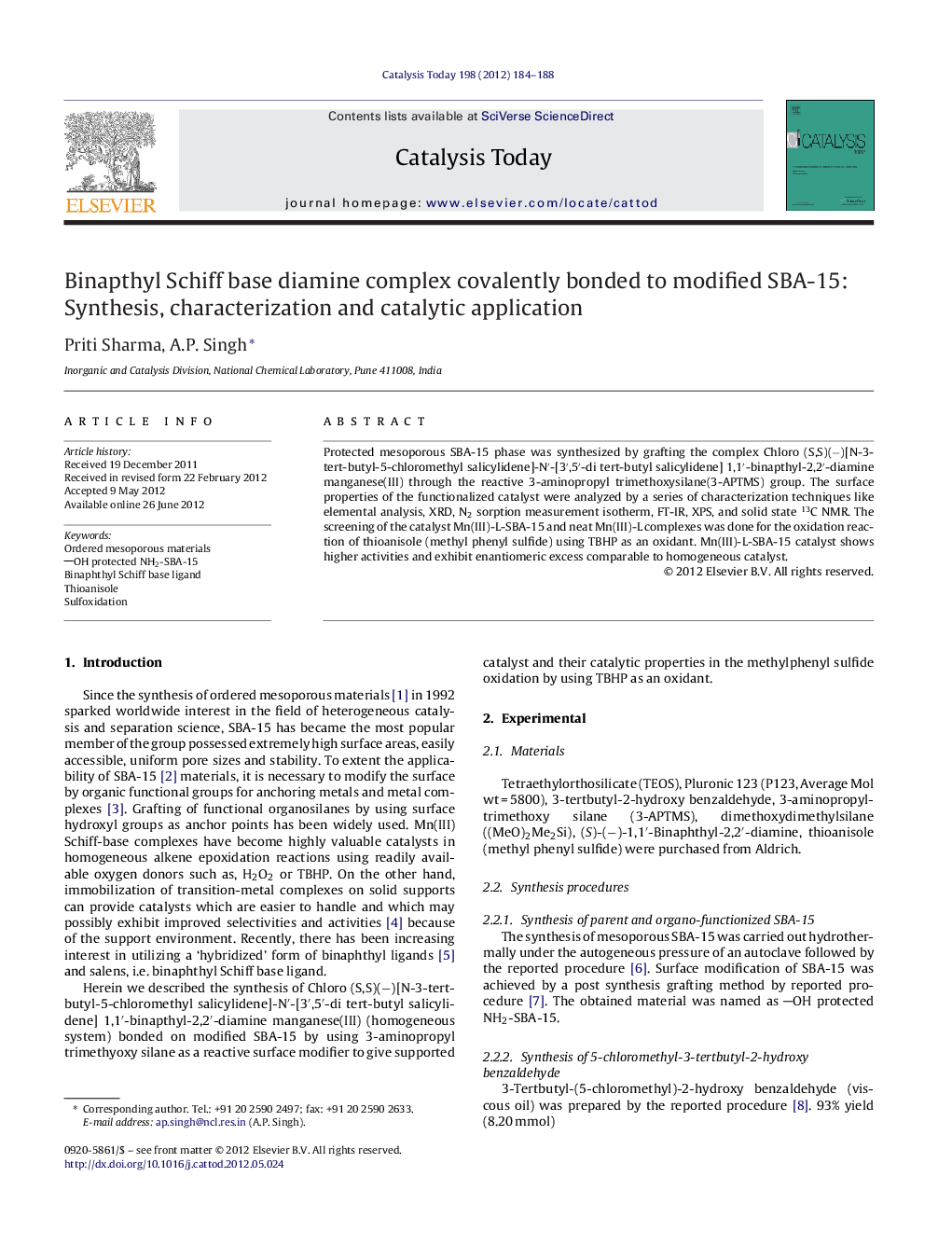
Mn(III)-L-SBA-15 complex. Mn(III)-L-SBA-15 = Chloro (S,S)(−)[N-3-tert-butyl-5-chloromethyl salicylidene]-N′-[3′,5′-di tert-butyl salicylidene] 1,1′-binapthyl-2,2′-diamine manganese(III) (homogeneous system) on modified SBA-15.Figure optionsDownload high-quality image (76 K)Download as PowerPoint slideHighlights
► The synthesis of mesoporous SBA-15 was carried out hydrothermally under the autogeneous pressure of an autoclave.
► Surface modification of SBA-15 was achieved by a post synthesis grafting method.
► Grafting of complex Chloro (S,S)(−)[N-3-tert-butyl-5-chloromethyl salicylidene]-N′-[3′,5′-di tert-butyl salicylidene] 1,1′-binapthyl-2,2′-diamine manganese(III) inside the OH protected NH2-SBA-15 was done by covalent bonding.
► Sulfoxidation reaction of methyl phenyl sulfide over Neat Mn(III) complex and well immobilized complex Mn(III)-L-SBA-15 were compared with and without catalyst condition.
Journal: Catalysis Today - Volume 198, Issue 1, 30 December 2012, Pages 184–188