| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 5526641 | 1547053 | 2017 | 10 صفحه PDF | دانلود رایگان |
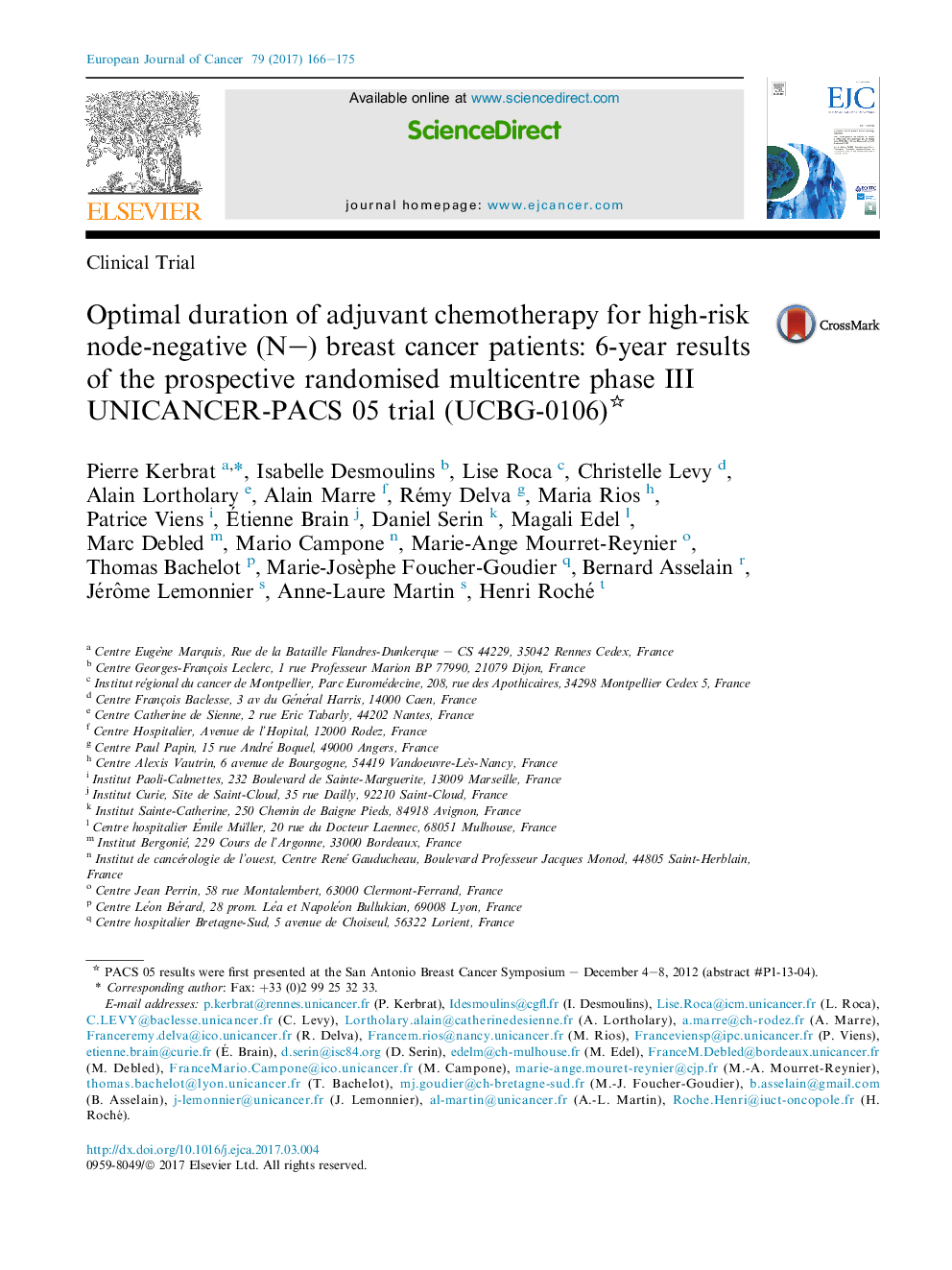
- 1516 high-risk breast cancer patients were randomised in 58 investigation centres.
- DFS rate at 5° years was 88.4% (4 FEC 100) versus 92.1% (6 FEC 100), HR = 1.18, P = .24.
- Grade 3 and 4 neutropenia were significantly more frequent with 6 FEC 100.
- The results suggest a trend toward better DFS and OS in the 6 FEC 100 arm.
- Assessment of high-risk patients must be more accurately defined.
PurposeOptimal duration of adjuvant chemotherapy in the treatment of early-stage breast cancer remained to be investigated rigorously for the standard regimens in widespread use in North America (doxorubicin/cyclophosphamide, AC) and Europe (5-fluorouracil/epirubicin/cyclophosphamide, FEC). Whether six cycles of FEC 100 present an advantage, or not, compared with only four cycles was tested directly in a phase III prospective multicentre trial.Patients and methodsBetween 2002 and 2006, 1515 women between 18 and 65°years of age, with node negative N(â) high-risk early-stage breast cancer, were included in the study following breast surgery and axillary lymph node dissection or procedure by sentinel node technique. Inclusion in the study required tumour size T â¥Â 1 cm and at least one of the high-risk factors: T > 2 cm, negative oestrogen receptor/progesterone receptor (ER- and PR-), Scarff-Bloom-Richardson (SBR) grade II or III and age â¤Â 35°years. Patients were randomly assigned to either six FEC 100 (Arm A) or four FEC 100 (Arm B). The trial was powered to detect an absolute difference â¥6% in disease-free survival (DFS) at 5°years.ResultsAt 6.1°years median follow-up, with 91 (12%) events recorded in Arm A versus 106 (14%) in Arm B, no statistically significant risk increase was associated with four versus six FEC 100: DFS (hazard ratio (HR) = 1.18; CI 95% [0.89-1.56], P = .24) and overall survival (OS) (HR = 1.39; CI 95% [0.91-2.13], P = .12).ConclusionDifferences in chemotherapy duration did not induce notably different outcomes in our cohort of high-risk patients.Clinical trial registry numberNCT00055679, Agence National de Sécurité du Médicament (ANSM) - France.
Journal: European Journal of Cancer - Volume 79, July 2017, Pages 166-175