| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 58949 | 1419429 | 2015 | 8 صفحه PDF | دانلود رایگان |
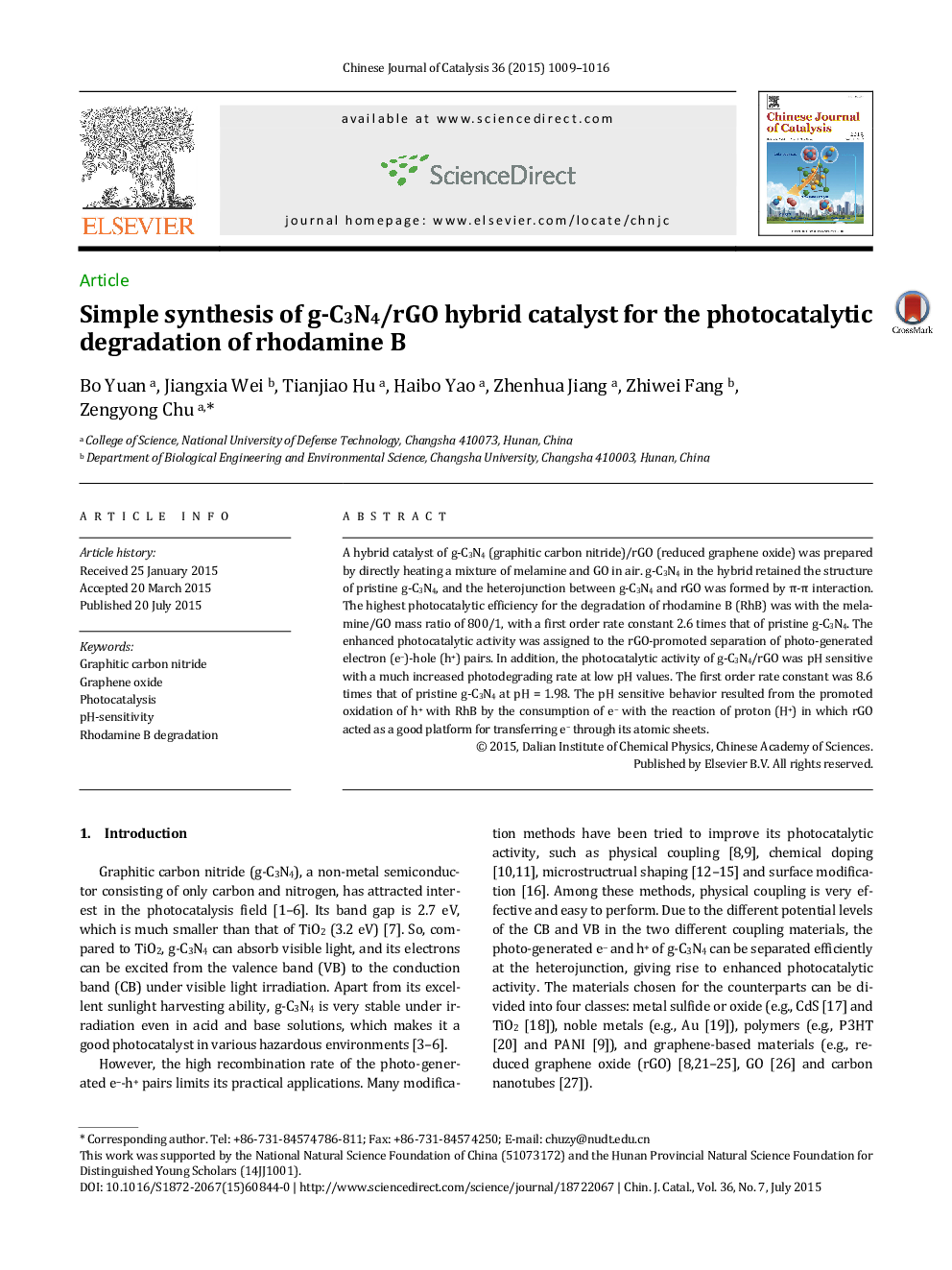
A hybrid catalyst of g-C3N4 (graphitic carbon nitride)/rGO (reduced graphene oxide) was prepared by directly heating a mixture of melamine and GO in air. g-C3N4 in the hybrid retained the structure of pristine g-C3N4, and the heterojunction between g-C3N4 and rGO was formed by π-π interaction. The highest photocatalytic efficiency for the degradation of rhodamine B (RhB) was with the melamine/GO mass ratio of 800/1, with a first order rate constant 2.6 times that of pristine g-C3N4. The enhanced photocatalytic activity was assigned to the rGO-promoted separation of photo-generated electron (e−)-hole (h+) pairs. In addition, the photocatalytic activity of g-C3N4/rGO was pH sensitive with a much increased photodegrading rate at low pH values. The first order rate constant was 8.6 times that of pristine g-C3N4 at pH = 1.98. The pH sensitive behavior resulted from the promoted oxidation of h+ with RhB by the consumption of e− with the reaction of proton (H+) in which rGO acted as a good platform for transferring e− through its atomic sheets.
Graphical AbstractA hybrid catalyst of g-C3N4/rGO was prepared by directly heating a mixture of melamine and graphene oxide in air, which showed superior photocatalytic activity in the degradation of RhB under acidic conditions.Figure optionsDownload as PowerPoint slide
Journal: Chinese Journal of Catalysis - Volume 36, Issue 7, July 2015, Pages 1009–1016