| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 608432 | 880591 | 2011 | 8 صفحه PDF | دانلود رایگان |
Mixed oxide TiO2–Fe2O3 bi-composites have been recognised as efficient and economical sorbents with great promise for arsenic removal from groundwater. In this study, we use a fast, simple and inexpensive synthesis method for this type of bi-composite and assess its adsorption performance. The kinetics of arsenate and phosphate adsorption onto the bi-composite are determined, demonstrating rapid and stable uptake of both oxy-anions over several days and with improved performance compared to the widely used TiO2 sorbent. A modified pseudo-second order rate equation is introduced, which allows the adsorption kinetics to be modelled as two simultaneous, parallel reaction pathways with separate kinetic parameters. This equation reproduces the experimental observations accurately across a wide range of timescales from minutes to days. Our experimental data agrees with previous interpretations of the adsorption mechanism including the formation of mono-dentate and bi-dentate inner-sphere surface complexes. The arsenate and phosphate uptake capacities of the bi-composite are reported. Equilibrium studies were conducted between pH 5 and 9 and interpreted within the Langmuir, Freundlich and Dubinin–Radushkevich isotherm models.
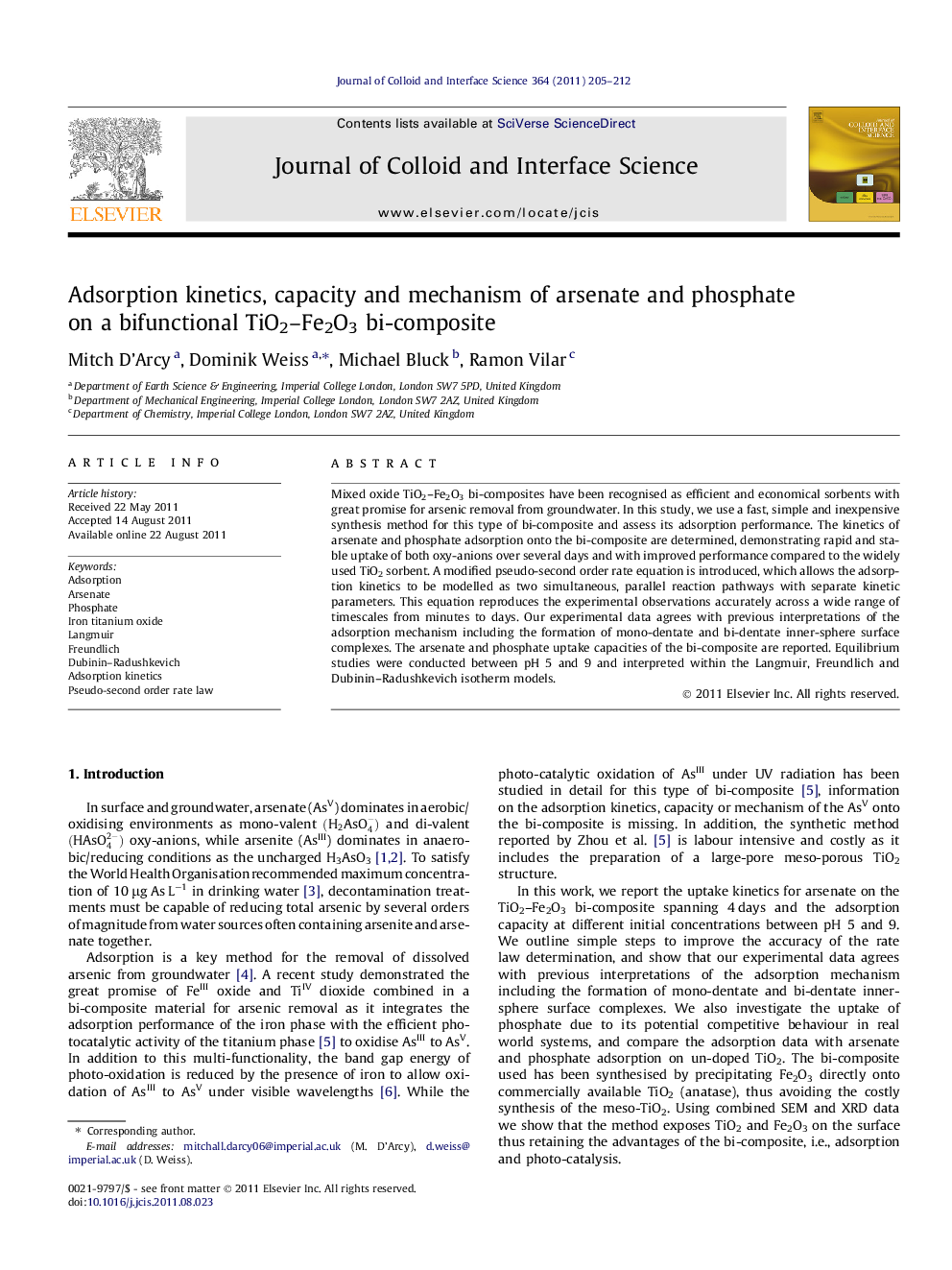
Illustration of the proposed adsorption binding mechanism of AsV onto the TiO2–Fe2O3 bi-composite, based on kinetic and adsorption isotherm modelling.Figure optionsDownload high-quality image (25 K)Download as PowerPoint slideHighlights
► Fast and simple method for the synthesis of TiO2–Fe2O3 bi-composites.
► As and P adsorbed within one minute and strongly bound over days.
► Modified rate equation models kinetics as two simultaneous reactions.
► Adsorption interpreted as parallel mono- and bi-dentate complexation.
► Adsorption capacity is 12 mg As g−1 in slightly acidic conditions.
Journal: Journal of Colloid and Interface Science - Volume 364, Issue 1, 1 December 2011, Pages 205–212