| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 609235 | 880618 | 2010 | 5 صفحه PDF | دانلود رایگان |
The decomposition reaction of the purple dye murexide in acidic media is used as a probe indicator for protons in nonionic microemulsions. The reaction kinetics primarily rely on the proton concentration and permit assessment of the proton activity in the nonionic microemulsions of water/cyclohexane/Igepal and water/heptane/Igepal. The experiments performed in the two microemulsions covered a wide range of water-to-oil mass fraction for the two systems. The kinetic runs were monitored under pseudo-first order conditions by the stopped-flow technique. The equilibrium constants for the formation of purpuric acid and the kinetic constants for the ensuing decomposition reaction fulfill a trend consistent with the micro compartmentalized nature of the multicomponent medium, and support the use of murexide as an indicator of the proton activity in microemulsions.
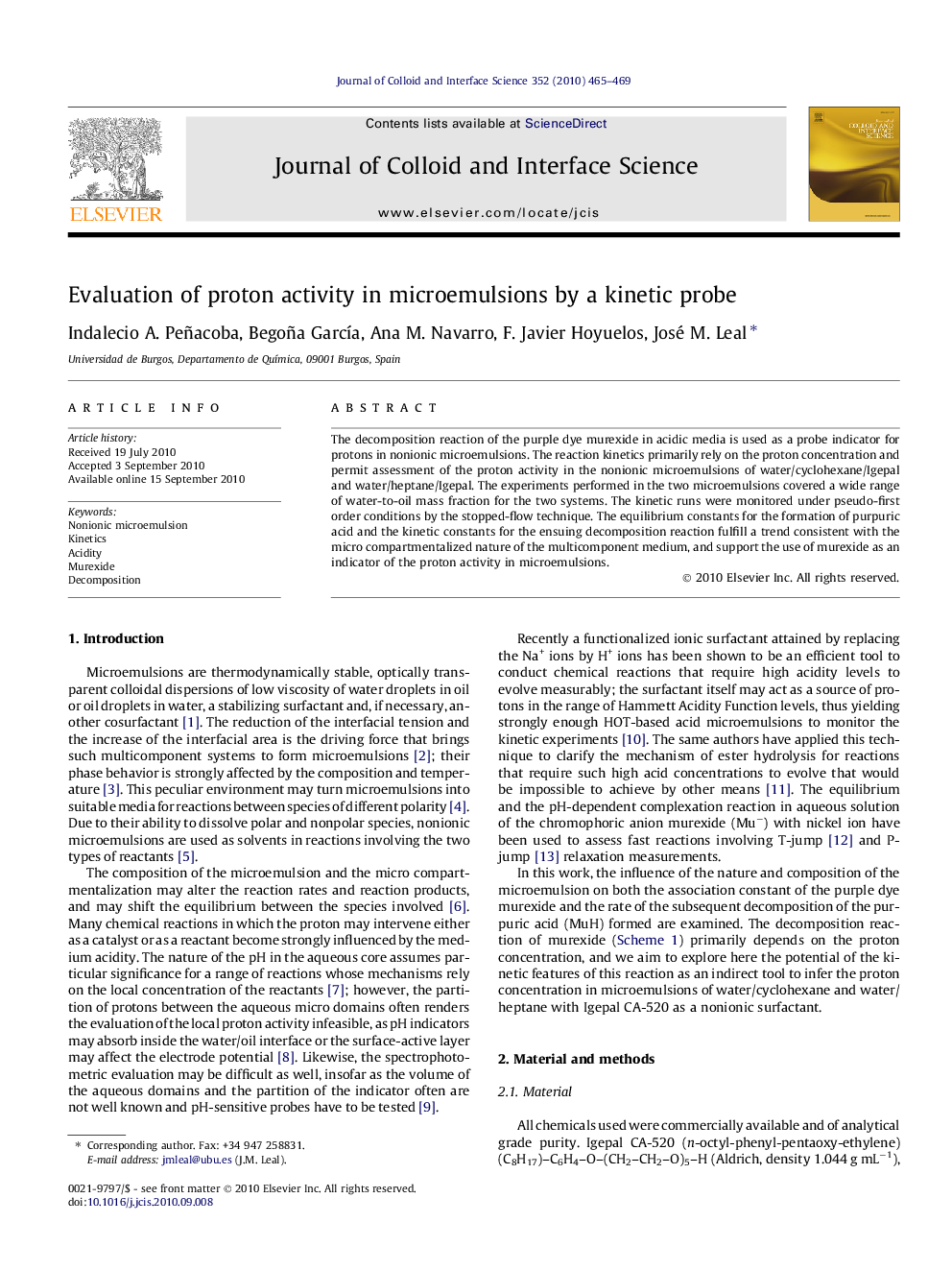
Variation of the relaxation constant as a function of the proton concentration for the decomposition reaction of murexide in nonionic microemulsion with Igepal CA-520.Figure optionsDownload high-quality image (25 K)Download as PowerPoint slideResearch highlights
► Murexide decomposes irreversibly in nonionic microemulsions.
► Heptane and cyclohexane permit to cover a wide range of water/oil content and microemulsion composition.
► The rate constants of the decomposition reaction of murexide primarily depend on the proton concentration and are indicative of the medium acidity in the microemulsions.
Journal: Journal of Colloid and Interface Science - Volume 352, Issue 2, 15 December 2010, Pages 465–469