| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 612237 | 880693 | 2007 | 7 صفحه PDF | دانلود رایگان |
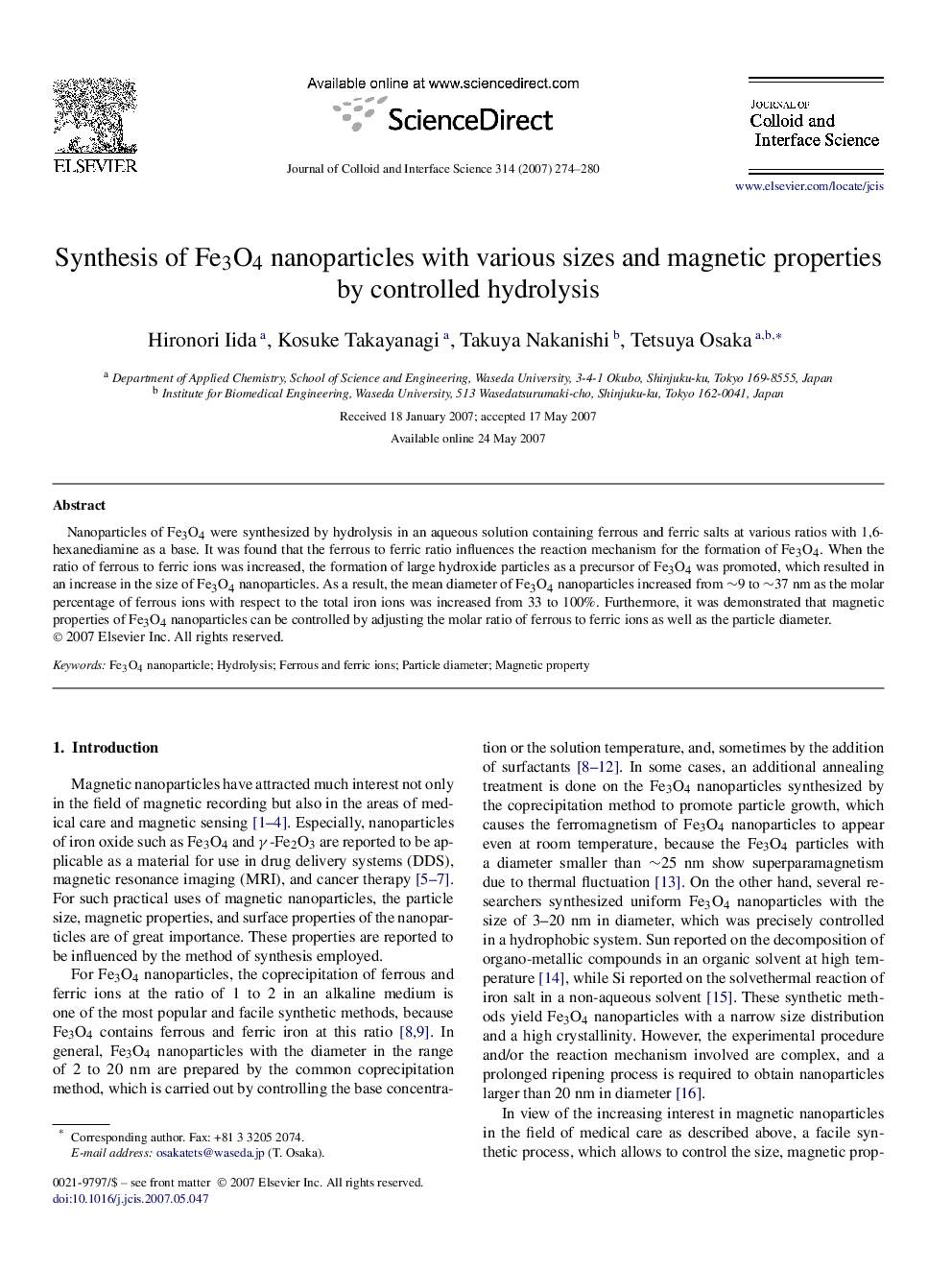
Nanoparticles of Fe3O4 were synthesized by hydrolysis in an aqueous solution containing ferrous and ferric salts at various ratios with 1,6-hexanediamine as a base. It was found that the ferrous to ferric ratio influences the reaction mechanism for the formation of Fe3O4. When the ratio of ferrous to ferric ions was increased, the formation of large hydroxide particles as a precursor of Fe3O4 was promoted, which resulted in an increase in the size of Fe3O4 nanoparticles. As a result, the mean diameter of Fe3O4 nanoparticles increased from ∼9 to ∼37 nm as the molar percentage of ferrous ions with respect to the total iron ions was increased from 33 to 100%. Furthermore, it was demonstrated that magnetic properties of Fe3O4 nanoparticles can be controlled by adjusting the molar ratio of ferrous to ferric ions as well as the particle diameter.
Fe3O4 nanoparticles with various sizes and magnetic properties were synthesized by controlling the ratio of ferrous and ferric ions in the hydrolysis reaction.Figure optionsDownload as PowerPoint slide
Journal: Journal of Colloid and Interface Science - Volume 314, Issue 1, 1 October 2007, Pages 274–280