| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 61383 | 47578 | 2012 | 10 صفحه PDF | دانلود رایگان |
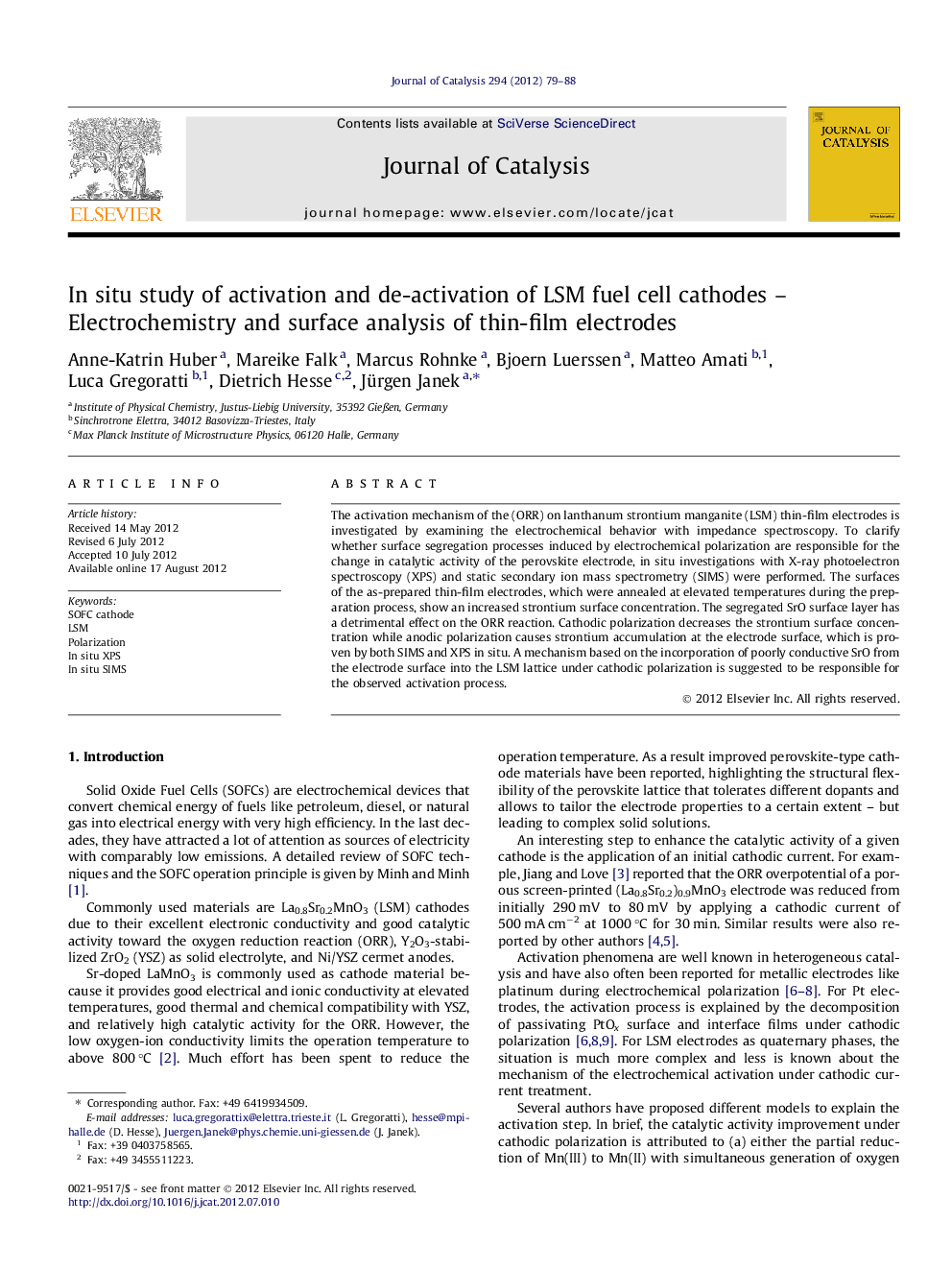
The activation mechanism of the (ORR) on lanthanum strontium manganite (LSM) thin-film electrodes is investigated by examining the electrochemical behavior with impedance spectroscopy. To clarify whether surface segregation processes induced by electrochemical polarization are responsible for the change in catalytic activity of the perovskite electrode, in situ investigations with X-ray photoelectron spectroscopy (XPS) and static secondary ion mass spectrometry (SIMS) were performed. The surfaces of the as-prepared thin-film electrodes, which were annealed at elevated temperatures during the preparation process, show an increased strontium surface concentration. The segregated SrO surface layer has a detrimental effect on the ORR reaction. Cathodic polarization decreases the strontium surface concentration while anodic polarization causes strontium accumulation at the electrode surface, which is proven by both SIMS and XPS in situ. A mechanism based on the incorporation of poorly conductive SrO from the electrode surface into the LSM lattice under cathodic polarization is suggested to be responsible for the observed activation process.
Activation and deactivation of mixed-conducting thin-film cathodes for high-temperature fuel cells were studied in situ with spatially resolved surface analysis techniques (X-ray photoelectron spectroscopy and static secondary ion mass spectrometry). A SrO surface layer that is formed during the annealing process or by applying an anodic potential has a detrimental effect on the oxygen reduction reaction. In contrast, cathodic polarization decreases the strontium surface concentration and an activation process is observed.Figure optionsDownload high-quality image (164 K)Download as PowerPoint slideHighlights
► Thin-film electrodes of LSM on YSZ (1 1 1) were prepared by pulsed laser deposition.
► In situ spectroscopy was used to investigate surface composition changes.
► The catalytic oxygen reduction reaction was investigated by impedance spectroscopy.
► Cathodic polarization accelerates the catalytic oxygen reduction reaction.
► A thermodynamic model based on the removal of poorly conductive SrO is proposed.
Journal: Journal of Catalysis - Volume 294, October 2012, Pages 79–88