| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 61594 | 47590 | 2011 | 14 صفحه PDF | دانلود رایگان |
The mechanism of the total oxidation of propane over alumina supported CuO, CeO2, and CuO–CeO2 is studied by means of Temporal Analysis of Products in the temperature range 623–873 K. A reaction scheme is proposed for the total oxidation of propane with O2, as well as for the separate reduction and oxidation steps. For the reduction of the catalyst with propane, four elementary steps are considered as kinetically significant: (1) reversible associative propane sorption, (2) irreversible dissociative propane adsorption involving a methylene C–H bond breaking and ultimately forming CO2*,s, (3) desorption of CO2*,s to CO2, and (4) dissociation of CO2*,s to CO*,s and O*,s, and recombination of CO*,s and O*,s to CO2*,s. For the oxidation of the catalyst with O2, two elementary steps are considered as kinetically significant: (1) reversible dissociative adsorption of O2 on two reduced active sites and (2) diffusion of lattice O atoms from the surface to the bulk and vice versa. An adequate description of the full mechanism, i.e., in the presence of propane and O2, can only be obtained by considering additional steps, which distinguish between lattice oxygen atoms at the surface, O*,s, and weakly bound oxygen atoms, Oweak. Apart from estimating the different kinetic parameters, a new approach to determine the initial concentration of reduced active sites is presented.CuO–CeO2/γ-Al2O3 is a more efficient total oxidation catalyst than the corresponding single metal oxides. The activation energy for the first C–H bond activation in propane over the CuO–CeO2/γ-Al2O3 amounts to 62 kJ mol−1 and is significantly lower than the activation energies over the single metal oxides, i.e., 95 kJ mol−1 for CuO/θ-Al2O3 and 126 kJ mol−1 for CeO2/γ-Al2O3. The redox activity of CuO–CeO2/γ-Al2O3 is created by the ability to reduce and re-oxidize both CuO and CeO2, which is enhanced by a strong interaction between these phases.
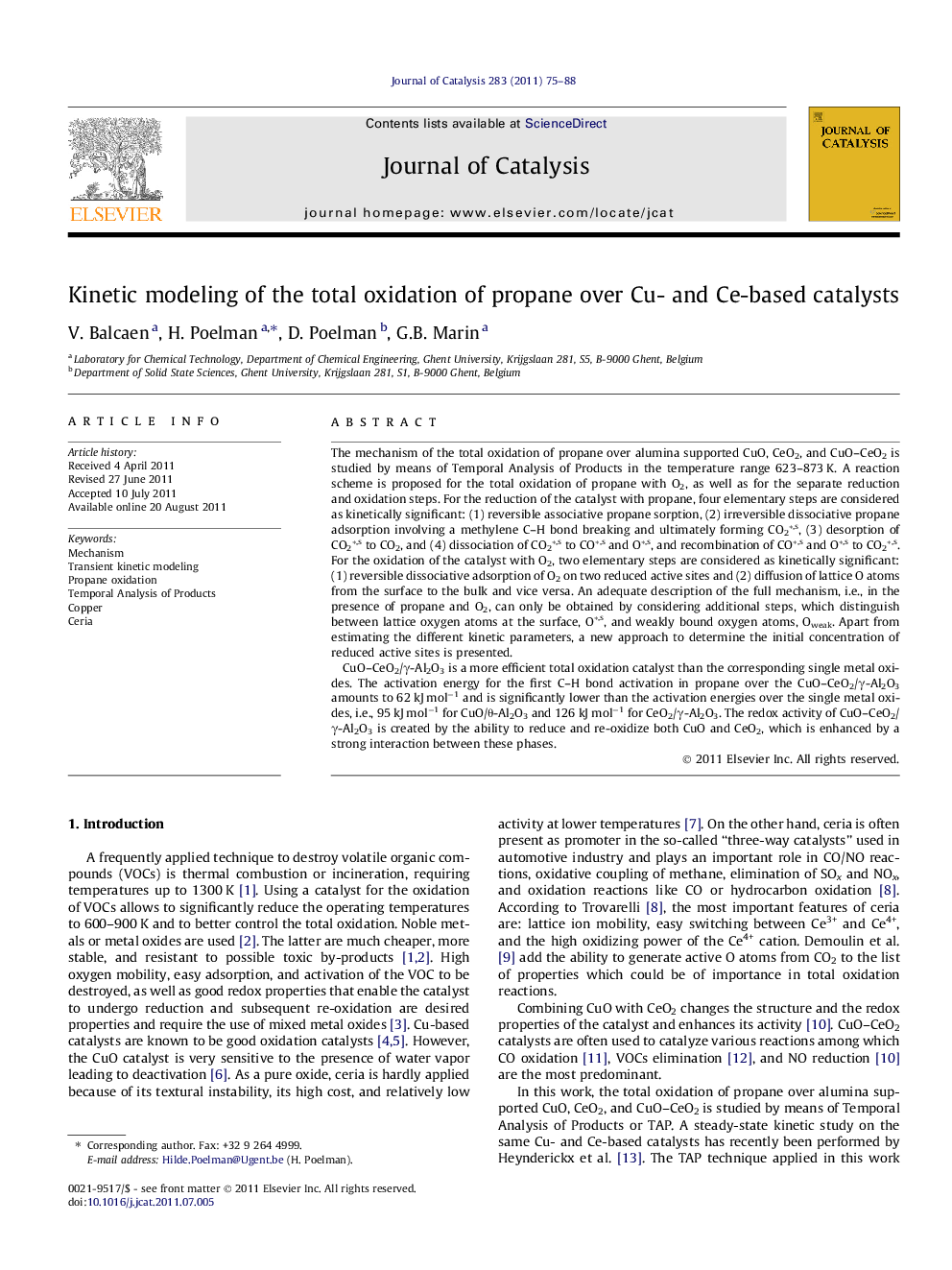
Propane total oxidation over CuO–CeO2/γ-Al2O3 occurs through a reaction network with kinetically significant steps for reduction, steps 5–7 and 17–21, and re-oxidation, steps 1–4, involving surface lattice oxygen, O*,s, and weakly bound oxygen, Oweak. Intermediate, kinetically non-significant steps are represented by dots between arrows. The constructed models describe adequately the redox processes, both combined and individually, from 623 K to 873 K.Figure optionsDownload high-quality image (65 K)Download as PowerPoint slideHighlights
► Mechanism of propane total oxidation studied by TAP.
► Comparison between alumina supported CuO, CeO2, and CuO–CeO2.
► Full description requires both surface lattice O and weakly bound O.
► New approach for determination of initial concentration of active sites.
► CuO–CeO2/γ-Al2O3 more efficient than the corresponding single metal oxides.
Journal: Journal of Catalysis - Volume 283, Issue 1, 6 October 2011, Pages 75–88