| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 6467345 | 1423252 | 2017 | 9 صفحه PDF | دانلود رایگان |
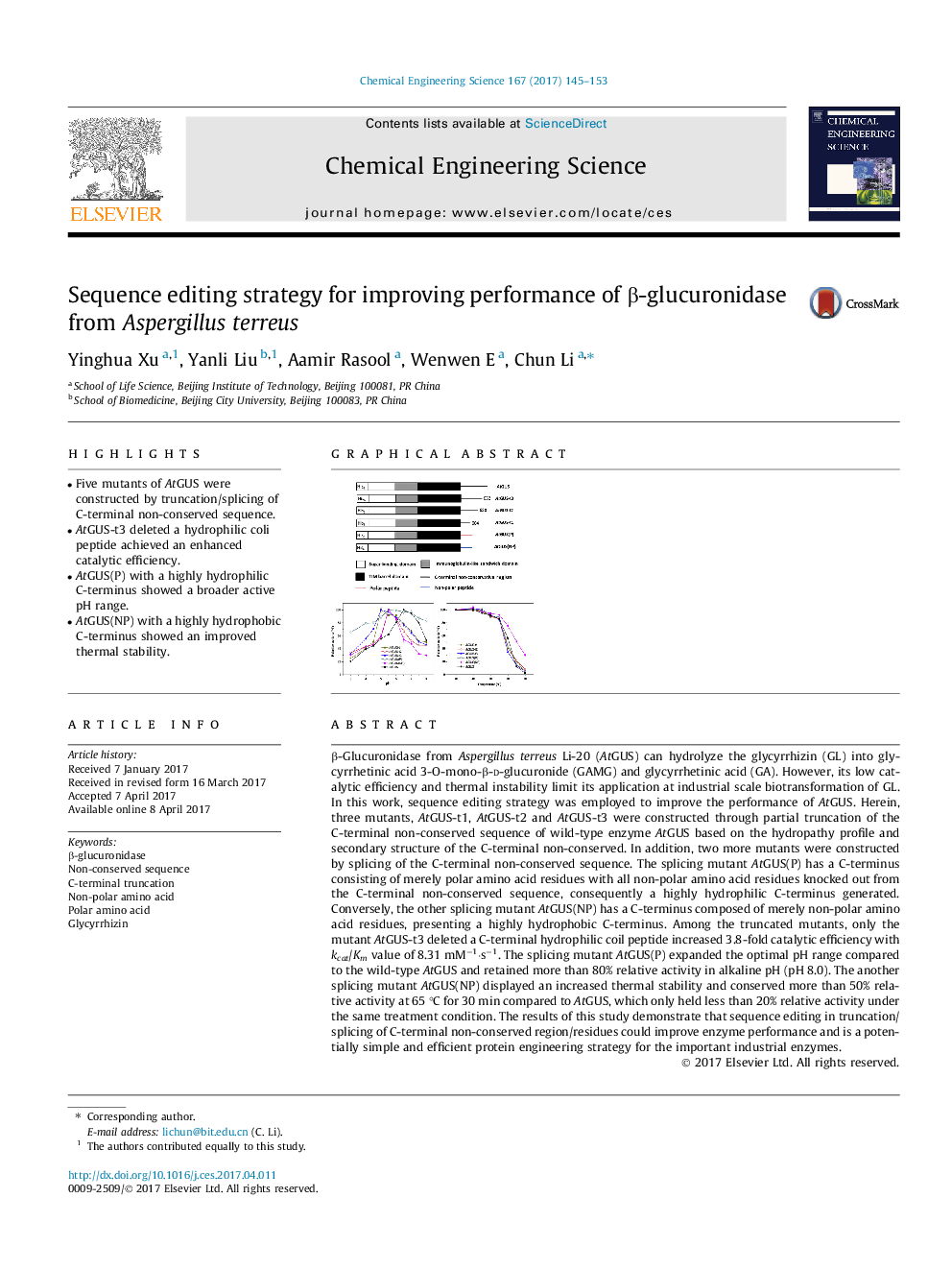
- Five mutants of AtGUS were constructed by truncation/splicing of C-terminal non-conserved sequence.
- AtGUS-t3 deleted a hydrophilic coli peptide achieved an enhanced catalytic efficiency.
- AtGUS(P) with a highly hydrophilic C-terminus showed a broader active pH range.
- AtGUS(NP) with a highly hydrophobic C-terminus showed an improved thermal stability.
β-Glucuronidase from Aspergillus terreus Li-20 (AtGUS) can hydrolyze the glycyrrhizin (GL) into glycyrrhetinic acid 3-O-mono-β-d-glucuronide (GAMG) and glycyrrhetinic acid (GA). However, its low catalytic efficiency and thermal instability limit its application at industrial scale biotransformation of GL. In this work, sequence editing strategy was employed to improve the performance of AtGUS. Herein, three mutants, AtGUS-t1, AtGUS-t2 and AtGUS-t3 were constructed through partial truncation of the C-terminal non-conserved sequence of wild-type enzyme AtGUS based on the hydropathy profile and secondary structure of the C-terminal non-conserved. In addition, two more mutants were constructed by splicing of the C-terminal non-conserved sequence. The splicing mutant AtGUS(P) has a C-terminus consisting of merely polar amino acid residues with all non-polar amino acid residues knocked out from the C-terminal non-conserved sequence, consequently a highly hydrophilic C-terminus generated. Conversely, the other splicing mutant AtGUS(NP) has a C-terminus composed of merely non-polar amino acid residues, presenting a highly hydrophobic C-terminus. Among the truncated mutants, only the mutant AtGUS-t3 deleted a C-terminal hydrophilic coil peptide increased 3.8-fold catalytic efficiency with kcat/Km value of 8.31 mMâ1·sâ1. The splicing mutant AtGUS(P) expanded the optimal pH range compared to the wild-type AtGUS and retained more than 80% relative activity in alkaline pH (pH 8.0). The another splicing mutant AtGUS(NP) displayed an increased thermal stability and conserved more than 50% relative activity at 65 °C for 30 min compared to AtGUS, which only held less than 20% relative activity under the same treatment condition. The results of this study demonstrate that sequence editing in truncation/splicing of C-terminal non-conserved region/residues could improve enzyme performance and is a potentially simple and efficient protein engineering strategy for the important industrial enzymes.
147
Journal: Chemical Engineering Science - Volume 167, 10 August 2017, Pages 145-153