| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 739984 | 1462090 | 2014 | 7 صفحه PDF | دانلود رایگان |
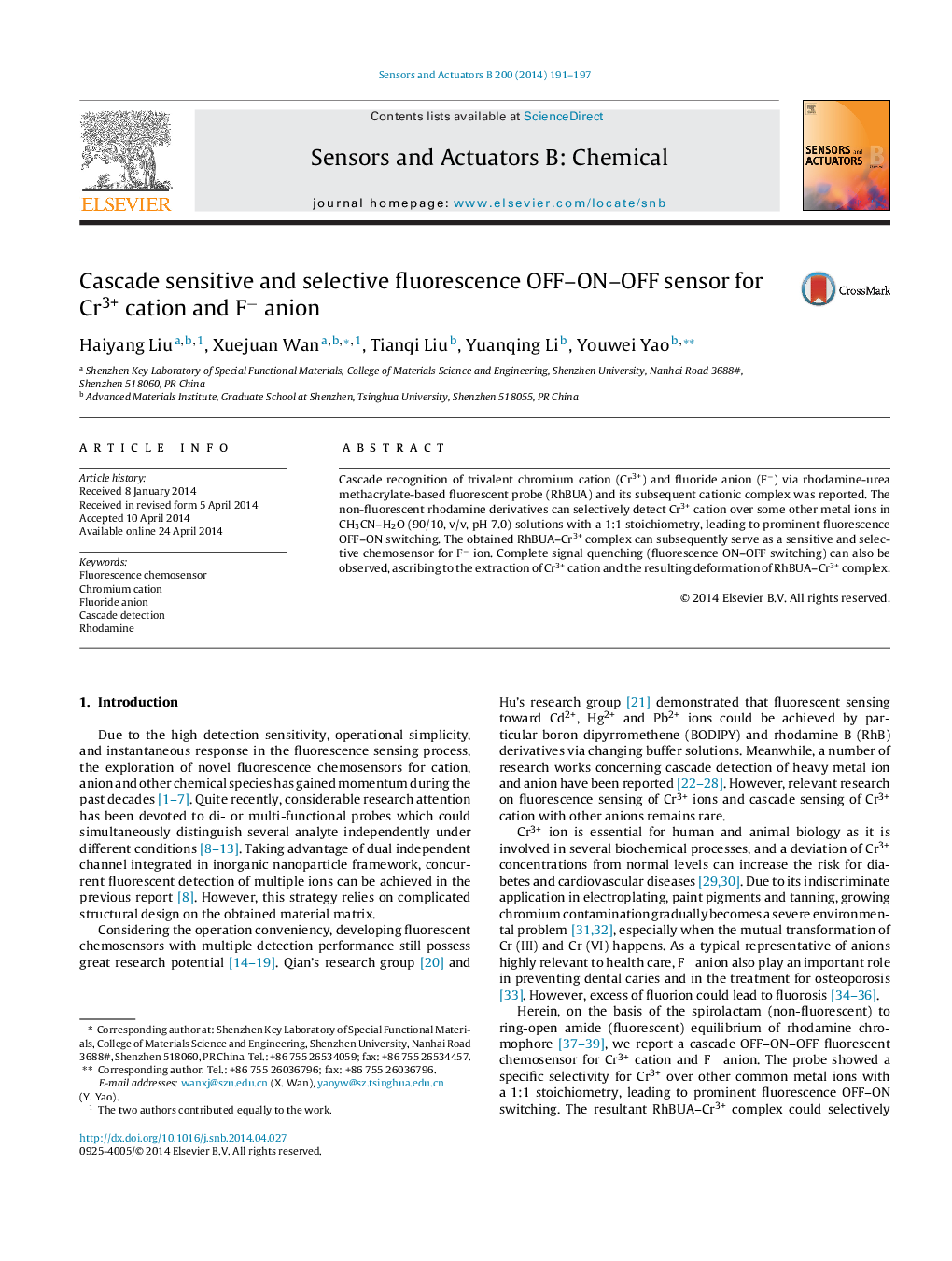
• Cascade fluorescence recognition of Cr3+ cation and F− anion was reported.
• Sensing of Cr3+ cation leads to prominent fluorescence OFF–ON switching.
• Sensing of F− exhibits complete ON–OFF fluorescence quenching.
• Detection limit for Cr3+ and F− are 5.5 × 10−8 M and 1.2 × 10−7 M, respectively.
Cascade recognition of trivalent chromium cation (Cr3+) and fluoride anion (F−) via rhodamine-urea methacrylate-based fluorescent probe (RhBUA) and its subsequent cationic complex was reported. The non-fluorescent rhodamine derivatives can selectively detect Cr3+ cation over some other metal ions in CH3CN–H2O (90/10, v/v, pH 7.0) solutions with a 1:1 stoichiometry, leading to prominent fluorescence OFF–ON switching. The obtained RhBUA–Cr3+ complex can subsequently serve as a sensitive and selective chemosensor for F− ion. Complete signal quenching (fluorescence ON–OFF switching) can also be observed, ascribing to the extraction of Cr3+ cation and the resulting deformation of RhBUA–Cr3+ complex.
Figure optionsDownload as PowerPoint slide
Journal: Sensors and Actuators B: Chemical - Volume 200, September 2014, Pages 191–197