| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1228801 | 1495222 | 2015 | 6 صفحه PDF | دانلود رایگان |
• The solvated H-bond complexes formed between DMF and the molecules are investigated.
• Absorption spectra of compounds were studied in some solvents of various polarities.
• A multiwavelength spectrometric was used to determine the pKa values of compounds.
• The investigated compounds showed positive solvatochromic behavior.
• The solvatochromism is due to solvent basicity and acidity properties of solute.
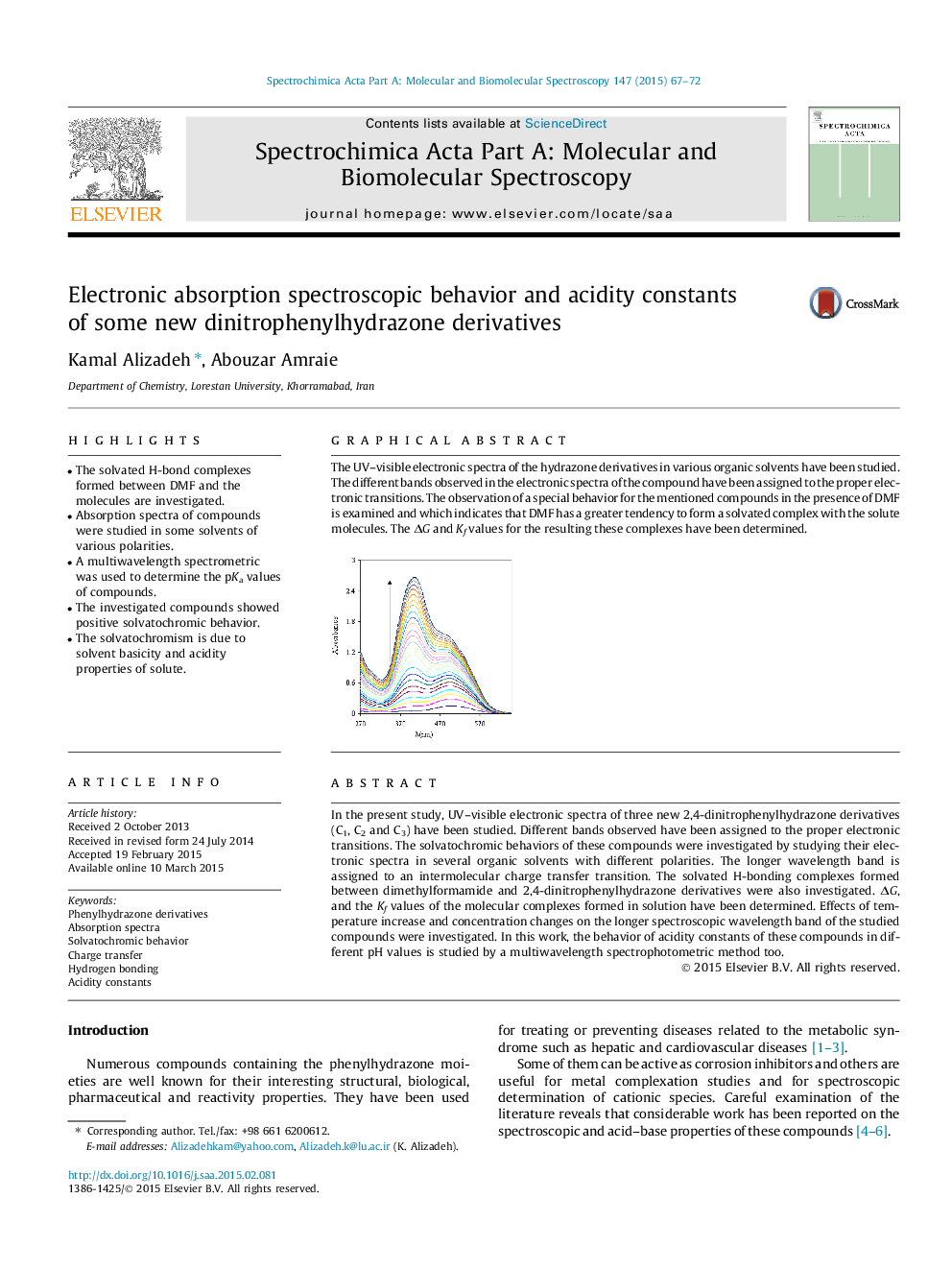
In the present study, UV–visible electronic spectra of three new 2,4-dinitrophenylhydrazone derivatives (C1, C2 and C3) have been studied. Different bands observed have been assigned to the proper electronic transitions. The solvatochromic behaviors of these compounds were investigated by studying their electronic spectra in several organic solvents with different polarities. The longer wavelength band is assigned to an intermolecular charge transfer transition. The solvated H-bonding complexes formed between dimethylformamide and 2,4-dinitrophenylhydrazone derivatives were also investigated. ΔG, and the Kf values of the molecular complexes formed in solution have been determined. Effects of temperature increase and concentration changes on the longer spectroscopic wavelength band of the studied compounds were investigated. In this work, the behavior of acidity constants of these compounds in different pH values is studied by a multiwavelength spectrophotometric method too.
The UV–visible electronic spectra of the hydrazone derivatives in various organic solvents have been studied. The different bands observed in the electronic spectra of the compound have been assigned to the proper electronic transitions. The observation of a special behavior for the mentioned compounds in the presence of DMF is examined and which indicates that DMF has a greater tendency to form a solvated complex with the solute molecules. The ΔG and Kf values for the resulting these complexes have been determined.Figure optionsDownload as PowerPoint slide
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 147, 5 August 2015, Pages 67–72