| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1229107 | 1495231 | 2015 | 7 صفحه PDF | دانلود رایگان |
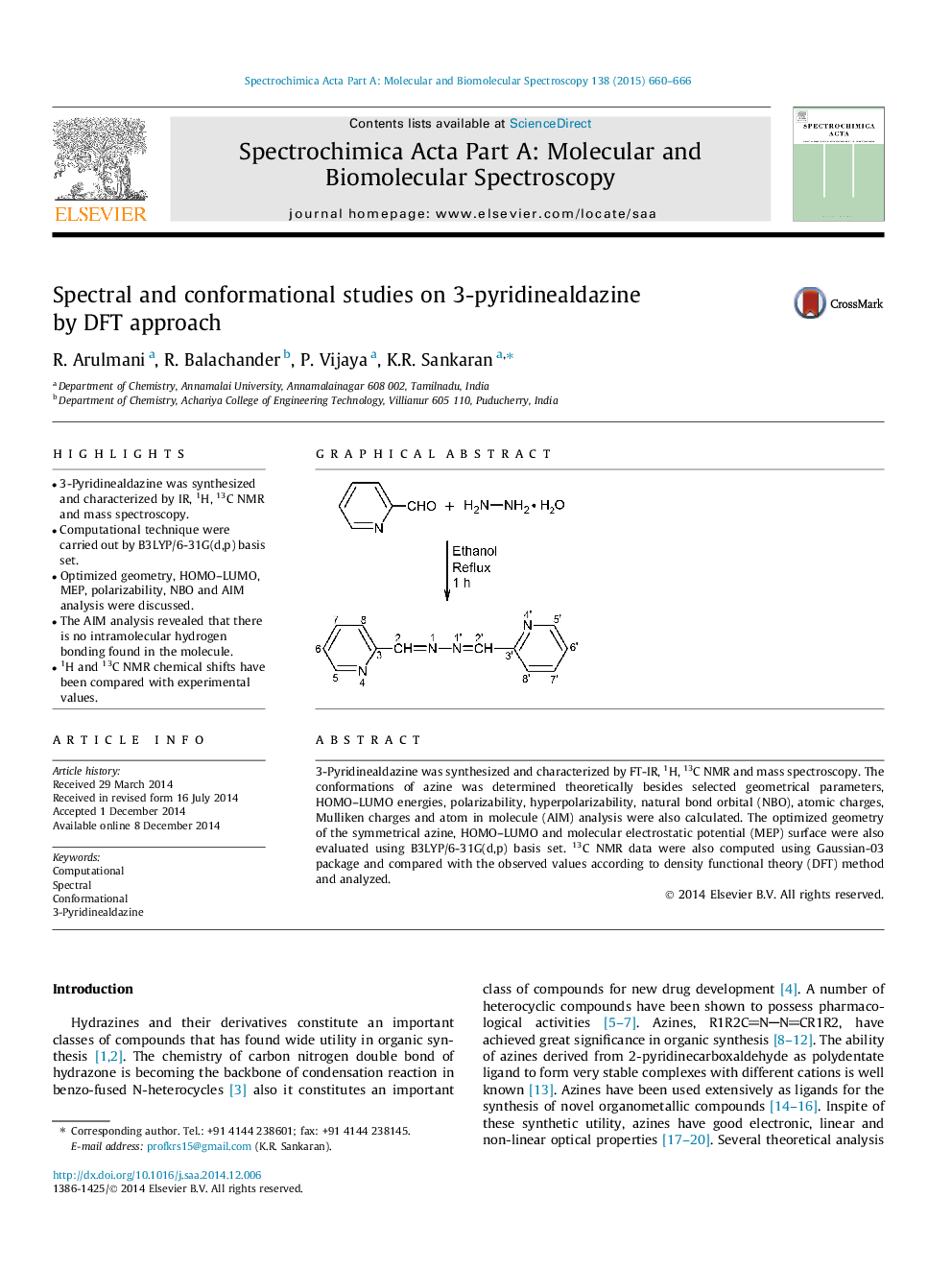
• 3-Pyridinealdazine was synthesized and characterized by IR, 1H, 13C NMR and mass spectroscopy.
• Computational technique were carried out by B3LYP/6-31G(d,p) basis set.
• Optimized geometry, HOMO–LUMO, MEP, polarizability, NBO and AIM analysis were discussed.
• The AIM analysis revealed that there is no intramolecular hydrogen bonding found in the molecule.
• 1H and 13C NMR chemical shifts have been compared with experimental values.
3-Pyridinealdazine was synthesized and characterized by FT-IR, 1H, 13C NMR and mass spectroscopy. The conformations of azine was determined theoretically besides selected geometrical parameters, HOMO–LUMO energies, polarizability, hyperpolarizability, natural bond orbital (NBO), atomic charges, Mulliken charges and atom in molecule (AIM) analysis were also calculated. The optimized geometry of the symmetrical azine, HOMO–LUMO and molecular electrostatic potential (MEP) surface were also evaluated using B3LYP/6-31G(d,p) basis set. 13C NMR data were also computed using Gaussian-03 package and compared with the observed values according to density functional theory (DFT) method and analyzed.
Figure optionsDownload as PowerPoint slide
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 138, 5 March 2015, Pages 660–666