| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1229235 | 1495232 | 2015 | 9 صفحه PDF | دانلود رایگان |
• Cationic porphyrin was firstly linked to a clinic drug, daunomycin.
• The porphyrin–daunomycin hybrids bind preferentially to G4 over i-motif DNA.
• Long-linked hybrids are more favorable in binding with G4 or i-motif structures.
Encouraged by the enormous importance attributed to the structure and function of human telomeric DNA, herein we focused our attention on the interaction of a serious of newly prepared porphyrin–daunomycin (Por–DNR) hybrids with the guanine-rich single-strand oligomer (G4) and the complementary cytosine-rich strand (i-motif). Various spectral methods such as absorption and fluorescence titration, surface-enhanced Raman and circular dichroism spectrum were integrated in the experiment and it was found that these Por–DNR hybrids could serve as prominent molecules to recognize G4 and i-motif. What is more, interesting results were obtained that the hybrids with longer flexible links are more favorable in binding with both G4 and i-motif than the hybrid with shorter linkage. These Por–DNR hybrids may help to develop new ideas in the research of human telomeric DNA with small molecules.
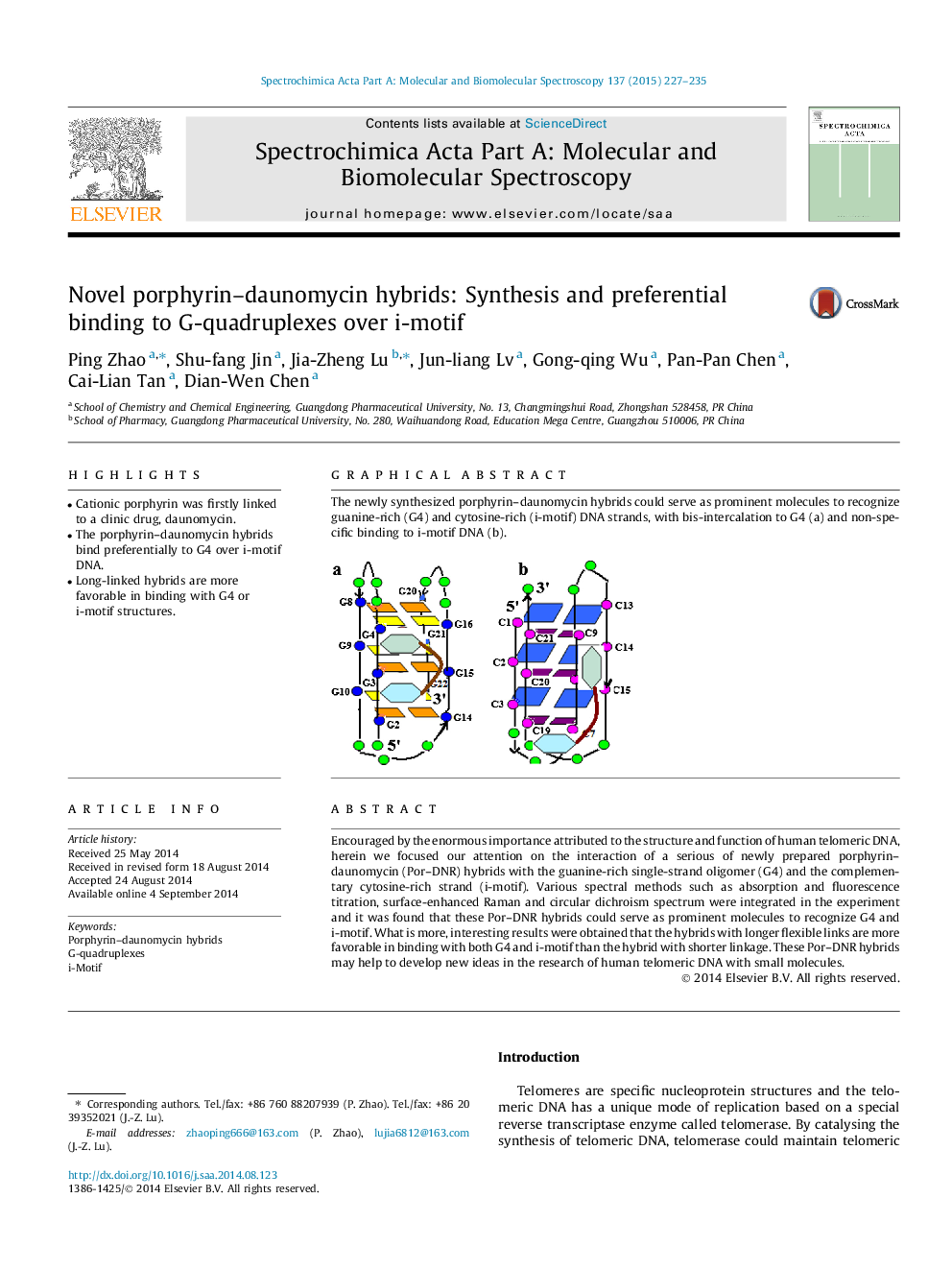
The newly synthesized porphyrin–daunomycin hybrids could serve as prominent molecules to recognize guanine-rich (G4) and cytosine-rich (i-motif) DNA strands, with bis-intercalation to G4 (a) and non-specific binding to i-motif DNA (b).Figure optionsDownload as PowerPoint slide
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 137, 25 February 2015, Pages 227–235