| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1229885 | 1495239 | 2014 | 12 صفحه PDF | دانلود رایگان |
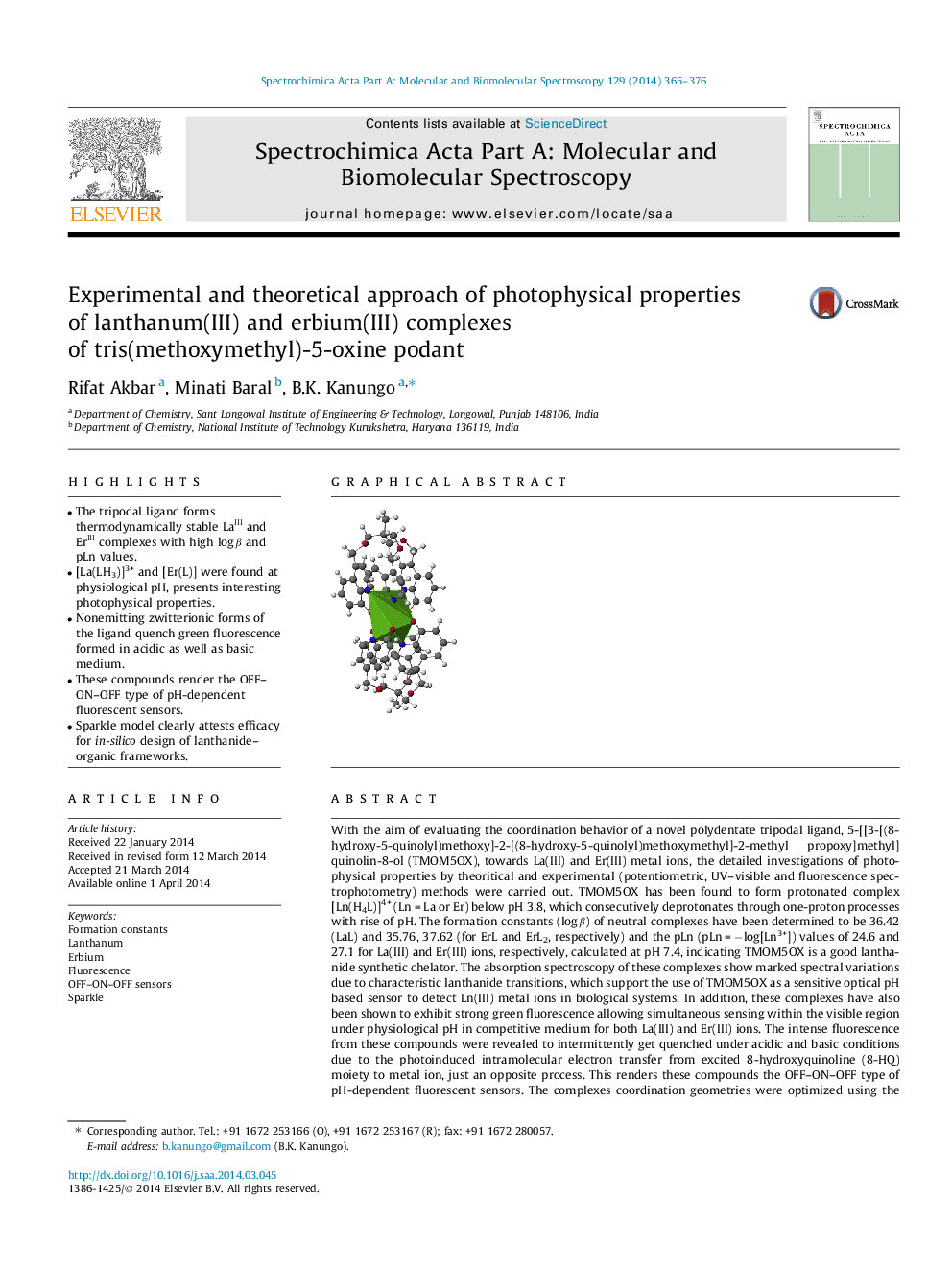
• The tripodal ligand forms thermodynamically stable LaIII and ErIII complexes with high log β and pLn values.
• [La(LH3)]3+ and [Er(L)] were found at physiological pH, presents interesting photophysical properties.
• Nonemitting zwitterionic forms of the ligand quench green fluorescence formed in acidic as well as basic medium.
• These compounds render the OFF–ON–OFF type of pH-dependent fluorescent sensors.
• Sparkle model clearly attests efficacy for in-silico design of lanthanide–organic frameworks.
With the aim of evaluating the coordination behavior of a novel polydentate tripodal ligand, 5-[[3-[(8-hydroxy-5-quinolyl)methoxy]-2-[(8-hydroxy-5-quinolyl)methoxymethyl]-2-methyl propoxy]methyl]quinolin-8-ol (TMOM5OX), towards La(III) and Er(III) metal ions, the detailed investigations of photophysical properties by theoritical and experimental (potentiometric, UV–visible and fluorescence spectrophotometry) methods were carried out. TMOM5OX has been found to form protonated complex [Ln(H4L)]4+ (Ln = La or Er) below pH 3.8, which consecutively deprotonates through one-proton processes with rise of pH. The formation constants (log β) of neutral complexes have been determined to be 36.42 (LaL) and 35.76, 37.62 (for ErL and ErL2, respectively) and the pLn (pLn = −log[Ln3+]) values of 24.6 and 27.1 for La(III) and Er(III) ions, respectively, calculated at pH 7.4, indicating TMOM5OX is a good lanthanide synthetic chelator. The absorption spectroscopy of these complexes show marked spectral variations due to characteristic lanthanide transitions, which support the use of TMOM5OX as a sensitive optical pH based sensor to detect Ln(III) metal ions in biological systems. In addition, these complexes have also been shown to exhibit strong green fluorescence allowing simultaneous sensing within the visible region under physiological pH in competitive medium for both La(III) and Er(III) ions. The intense fluorescence from these compounds were revealed to intermittently get quenched under acidic and basic conditions due to the photoinduced intramolecular electron transfer from excited 8-hydroxyquinoline (8-HQ) moiety to metal ion, just an opposite process. This renders these compounds the OFF–ON–OFF type of pH-dependent fluorescent sensors. The complexes coordination geometries were optimized using the sparkle/PM6 model and the theoretical spectrophotometric studies were carried out in order to validate the experimental findings, based on ZINDO/S methodology at configuration interaction with single excitations (CIS) level. These results clearly attest for the efficacy of the theoretical models employed in all calculations and create new interesting possibilities for the design in-silico of novel and highly efficient lanthanide–organic frameworks.
Figure optionsDownload as PowerPoint slide
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 129, 14 August 2014, Pages 365–376