| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1229943 | 1495242 | 2014 | 6 صفحه PDF | دانلود رایگان |
• A wardite mineral sample from Lavra Da Ilha, Minas Gerais, Brazil was analysed.
• Using SEM with EDX and vibrational spectroscopy.
• The calculated formula is (Na0.97Ca0.03)Σ1.00Al3(PO4)2(OH)4⋅2(H2O).
• Observation of multiple bands supports the concept of non-equivalent phosphate units in the structure.
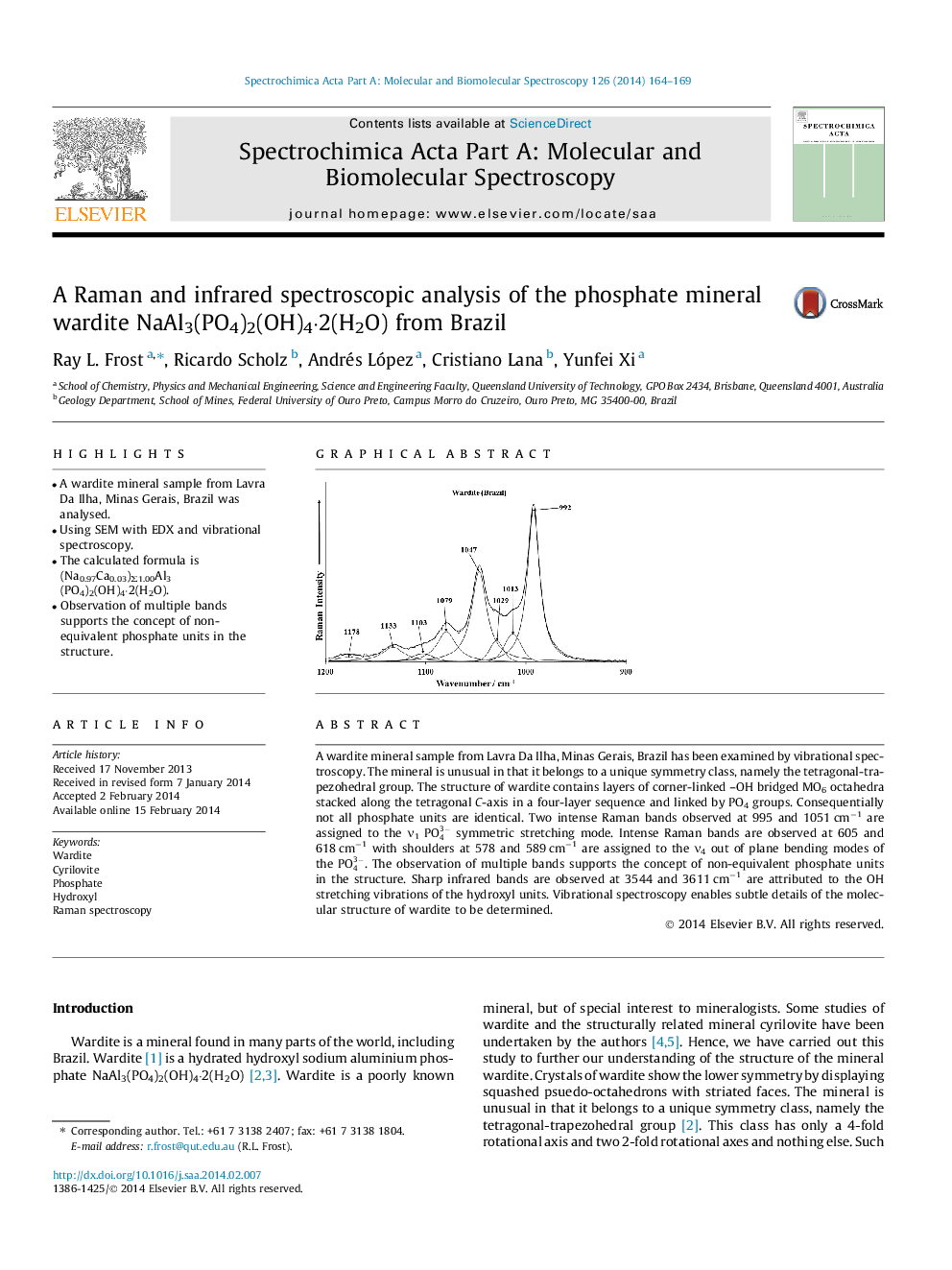
A wardite mineral sample from Lavra Da Ilha, Minas Gerais, Brazil has been examined by vibrational spectroscopy. The mineral is unusual in that it belongs to a unique symmetry class, namely the tetragonal-trapezohedral group. The structure of wardite contains layers of corner-linked –OH bridged MO6 octahedra stacked along the tetragonal C-axis in a four-layer sequence and linked by PO4 groups. Consequentially not all phosphate units are identical. Two intense Raman bands observed at 995 and 1051 cm−1 are assigned to the ν1PO43- symmetric stretching mode. Intense Raman bands are observed at 605 and 618 cm−1 with shoulders at 578 and 589 cm−1 are assigned to the ν4 out of plane bending modes of the PO43-. The observation of multiple bands supports the concept of non-equivalent phosphate units in the structure. Sharp infrared bands are observed at 3544 and 3611 cm−1 are attributed to the OH stretching vibrations of the hydroxyl units. Vibrational spectroscopy enables subtle details of the molecular structure of wardite to be determined.
Figure optionsDownload as PowerPoint slide
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 126, 21 May 2014, Pages 164–169