| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1229951 | 1495242 | 2014 | 10 صفحه PDF | دانلود رایگان |
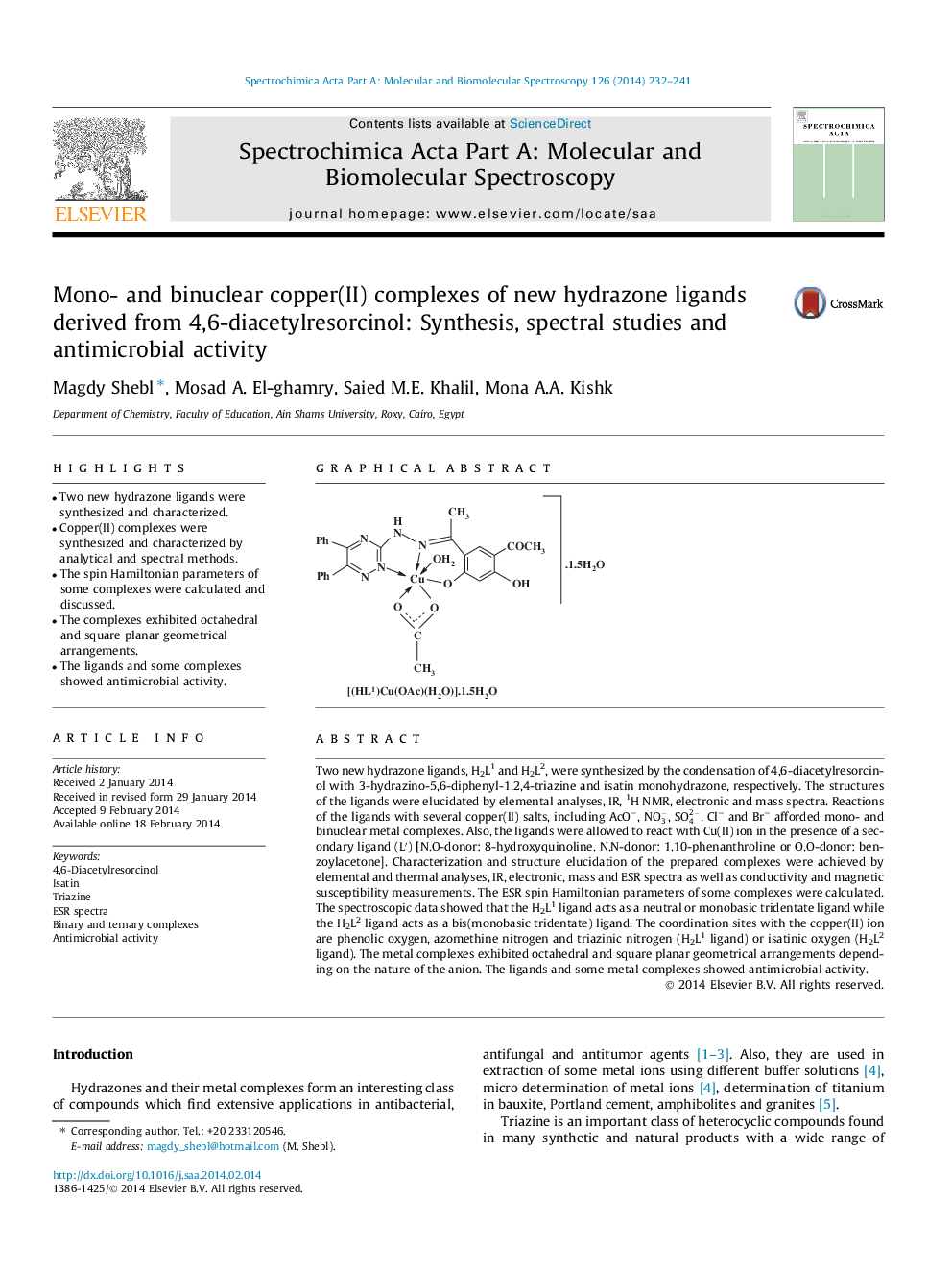
• Two new hydrazone ligands were synthesized and characterized.
• Copper(II) complexes were synthesized and characterized by analytical and spectral methods.
• The spin Hamiltonian parameters of some complexes were calculated and discussed.
• The complexes exhibited octahedral and square planar geometrical arrangements.
• The ligands and some complexes showed antimicrobial activity.
Two new hydrazone ligands, H2L1 and H2L2, were synthesized by the condensation of 4,6-diacetylresorcinol with 3-hydrazino-5,6-diphenyl-1,2,4-triazine and isatin monohydrazone, respectively. The structures of the ligands were elucidated by elemental analyses, IR, 1H NMR, electronic and mass spectra. Reactions of the ligands with several copper(II) salts, including AcO−, NO3-, SO42-, Cl− and Br− afforded mono- and binuclear metal complexes. Also, the ligands were allowed to react with Cu(II) ion in the presence of a secondary ligand (L′) [N,O-donor; 8-hydroxyquinoline, N,N-donor; 1,10-phenanthroline or O,O-donor; benzoylacetone]. Characterization and structure elucidation of the prepared complexes were achieved by elemental and thermal analyses, IR, electronic, mass and ESR spectra as well as conductivity and magnetic susceptibility measurements. The ESR spin Hamiltonian parameters of some complexes were calculated. The spectroscopic data showed that the H2L1 ligand acts as a neutral or monobasic tridentate ligand while the H2L2 ligand acts as a bis(monobasic tridentate) ligand. The coordination sites with the copper(II) ion are phenolic oxygen, azomethine nitrogen and triazinic nitrogen (H2L1 ligand) or isatinic oxygen (H2L2 ligand). The metal complexes exhibited octahedral and square planar geometrical arrangements depending on the nature of the anion. The ligands and some metal complexes showed antimicrobial activity.
Figure optionsDownload as PowerPoint slide
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 126, 21 May 2014, Pages 232–241