| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1229964 | 1495242 | 2014 | 6 صفحه PDF | دانلود رایگان |
• The polarized IR spectra of 4Br35DMPz and 34DMPAA were quantitatively analyzed.
• The in- and inter-chain vibrational exciton coupling mechanisms were identified.
• The origin of the temperature effects in the spectra was explained.
• The H/D isotopic self-organization effects were recognized.
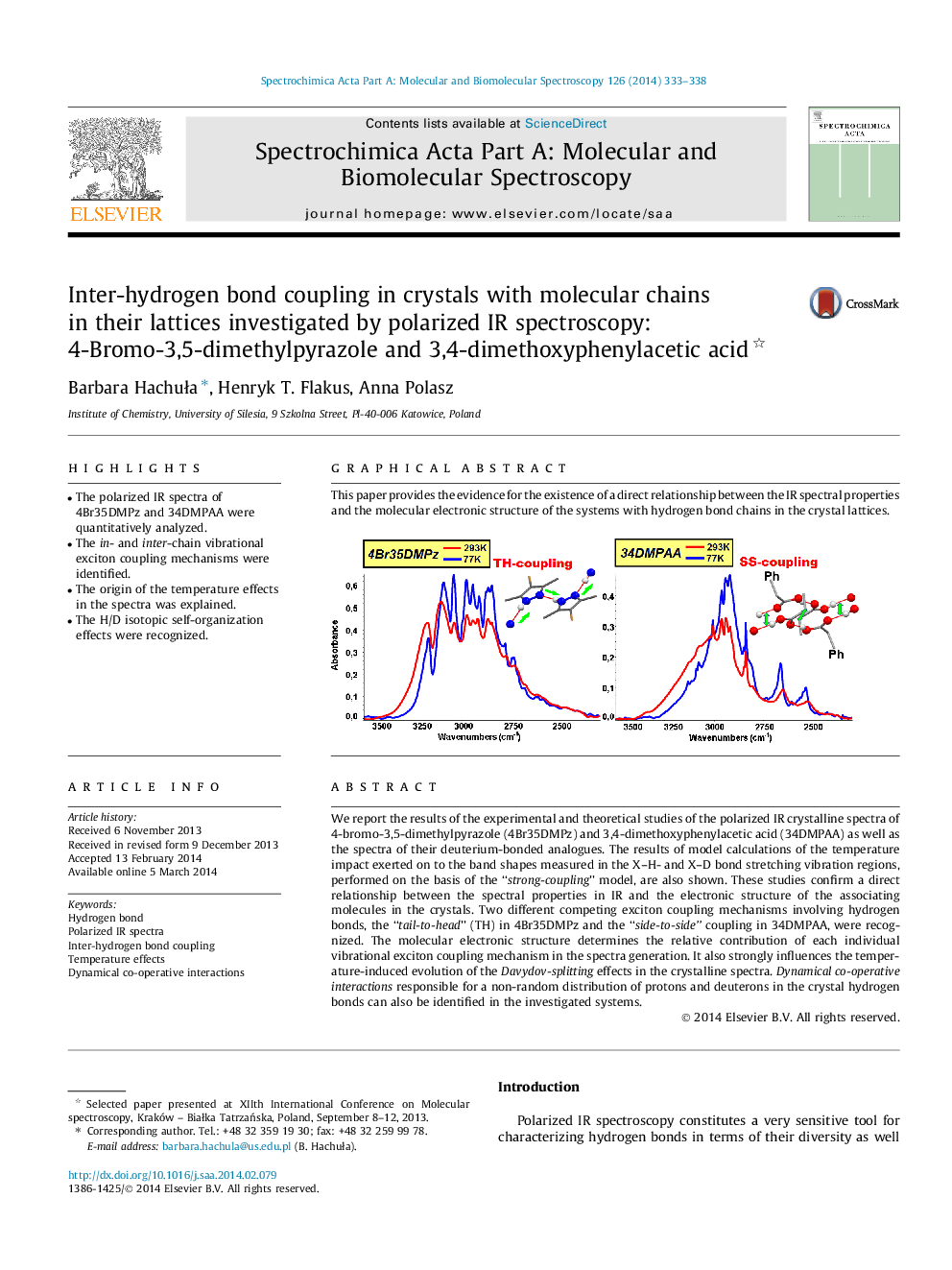
We report the results of the experimental and theoretical studies of the polarized IR crystalline spectra of 4-bromo-3,5-dimethylpyrazole (4Br35DMPz) and 3,4-dimethoxyphenylacetic acid (34DMPAA) as well as the spectra of their deuterium-bonded analogues. The results of model calculations of the temperature impact exerted on to the band shapes measured in the X–H- and X–D bond stretching vibration regions, performed on the basis of the “strong-coupling” model, are also shown. These studies confirm a direct relationship between the spectral properties in IR and the electronic structure of the associating molecules in the crystals. Two different competing exciton coupling mechanisms involving hydrogen bonds, the “tail-to-head” (TH) in 4Br35DMPz and the “side-to-side” coupling in 34DMPAA, were recognized. The molecular electronic structure determines the relative contribution of each individual vibrational exciton coupling mechanism in the spectra generation. It also strongly influences the temperature-induced evolution of the Davydov-splitting effects in the crystalline spectra. Dynamical co-operative interactions responsible for a non-random distribution of protons and deuterons in the crystal hydrogen bonds can also be identified in the investigated systems.
This paper provides the evidence for the existence of a direct relationship between the IR spectral properties and the molecular electronic structure of the systems with hydrogen bond chains in the crystal lattices.Figure optionsDownload as PowerPoint slide
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 126, 21 May 2014, Pages 333–338