| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1230480 | 1495247 | 2014 | 6 صفحه PDF | دانلود رایگان |
• A halogen-substituted Salen-type bisoxime has been synthesized firstly.
• A new complex [ZnL(H2O)2]n has been synthesized and characterized structurally.
• The complex forms a self-assembling infinite dual metal–water chain-like structure.
• Some new results are very important to coordination and supramolecular chemistry.
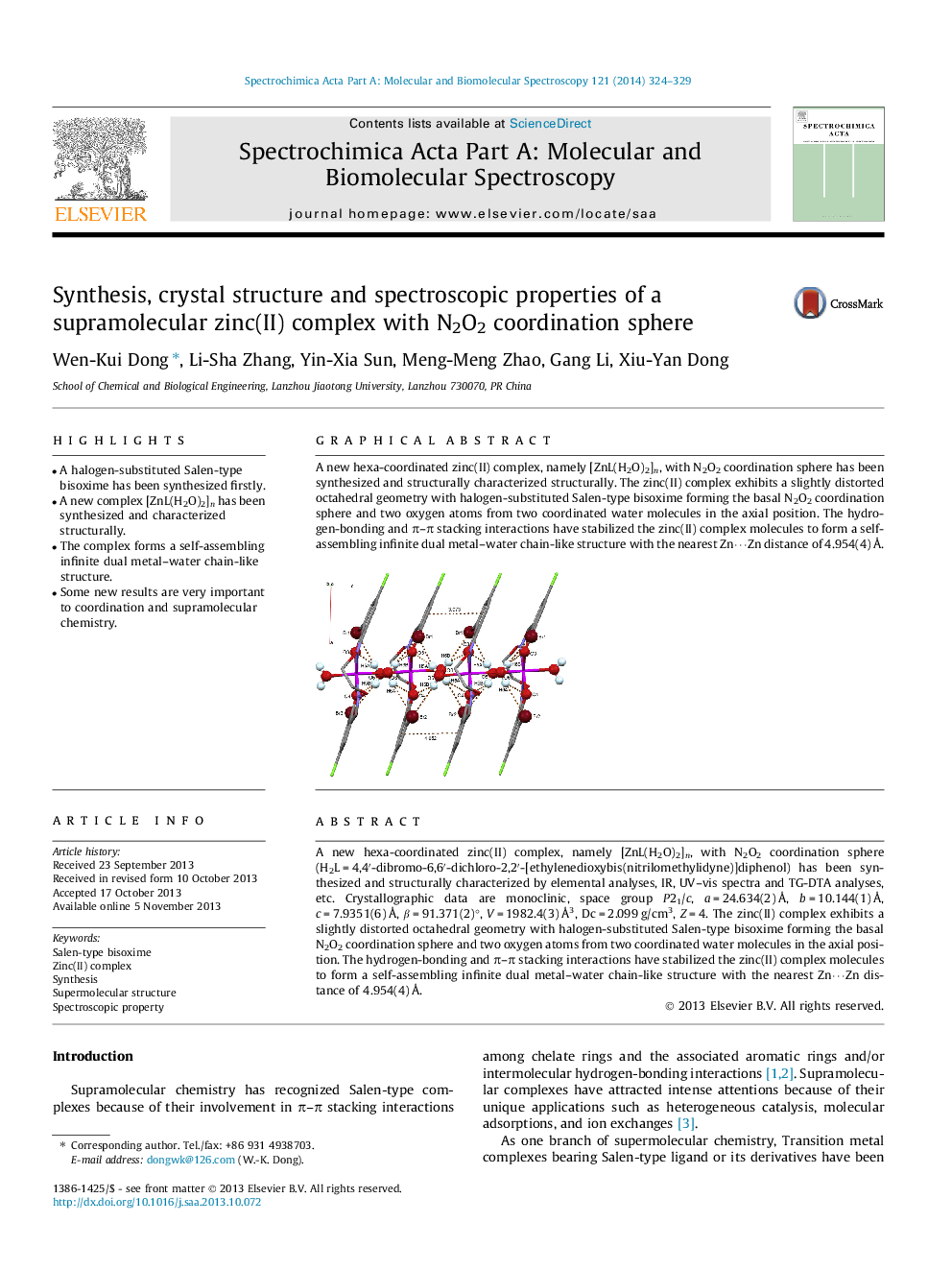
A new hexa-coordinated zinc(II) complex, namely [ZnL(H2O)2]n, with N2O2 coordination sphere (H2L = 4,4′-dibromo-6,6′-dichloro-2,2′-[ethylenedioxybis(nitrilomethylidyne)]diphenol) has been synthesized and structurally characterized by elemental analyses, IR, UV–vis spectra and TG-DTA analyses, etc. Crystallographic data are monoclinic, space group P21/c, a = 24.634(2) Å, b = 10.144(1) Å, c = 7.9351(6) Å, β = 91.371(2)°, V = 1982.4(3) Å3, Dc = 2.099 g/cm3, Z = 4. The zinc(II) complex exhibits a slightly distorted octahedral geometry with halogen-substituted Salen-type bisoxime forming the basal N2O2 coordination sphere and two oxygen atoms from two coordinated water molecules in the axial position. The hydrogen-bonding and π–π stacking interactions have stabilized the zinc(II) complex molecules to form a self-assembling infinite dual metal–water chain-like structure with the nearest Zn⋯Zn distance of 4.954(4) Å.
A new hexa-coordinated zinc(II) complex, namely [ZnL(H2O)2]n, with N2O2 coordination sphere has been synthesized and structurally characterized structurally. The zinc(II) complex exhibits a slightly distorted octahedral geometry with halogen-substituted Salen-type bisoxime forming the basal N2O2 coordination sphere and two oxygen atoms from two coordinated water molecules in the axial position. The hydrogen-bonding and π–π stacking interactions have stabilized the zinc(II) complex molecules to form a self-assembling infinite dual metal–water chain-like structure with the nearest Zn⋯Zn distance of 4.954(4) Å.Figure optionsDownload as PowerPoint slide
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 121, 5 March 2014, Pages 324–329