| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1230690 | 1495261 | 2013 | 8 صفحه PDF | دانلود رایگان |
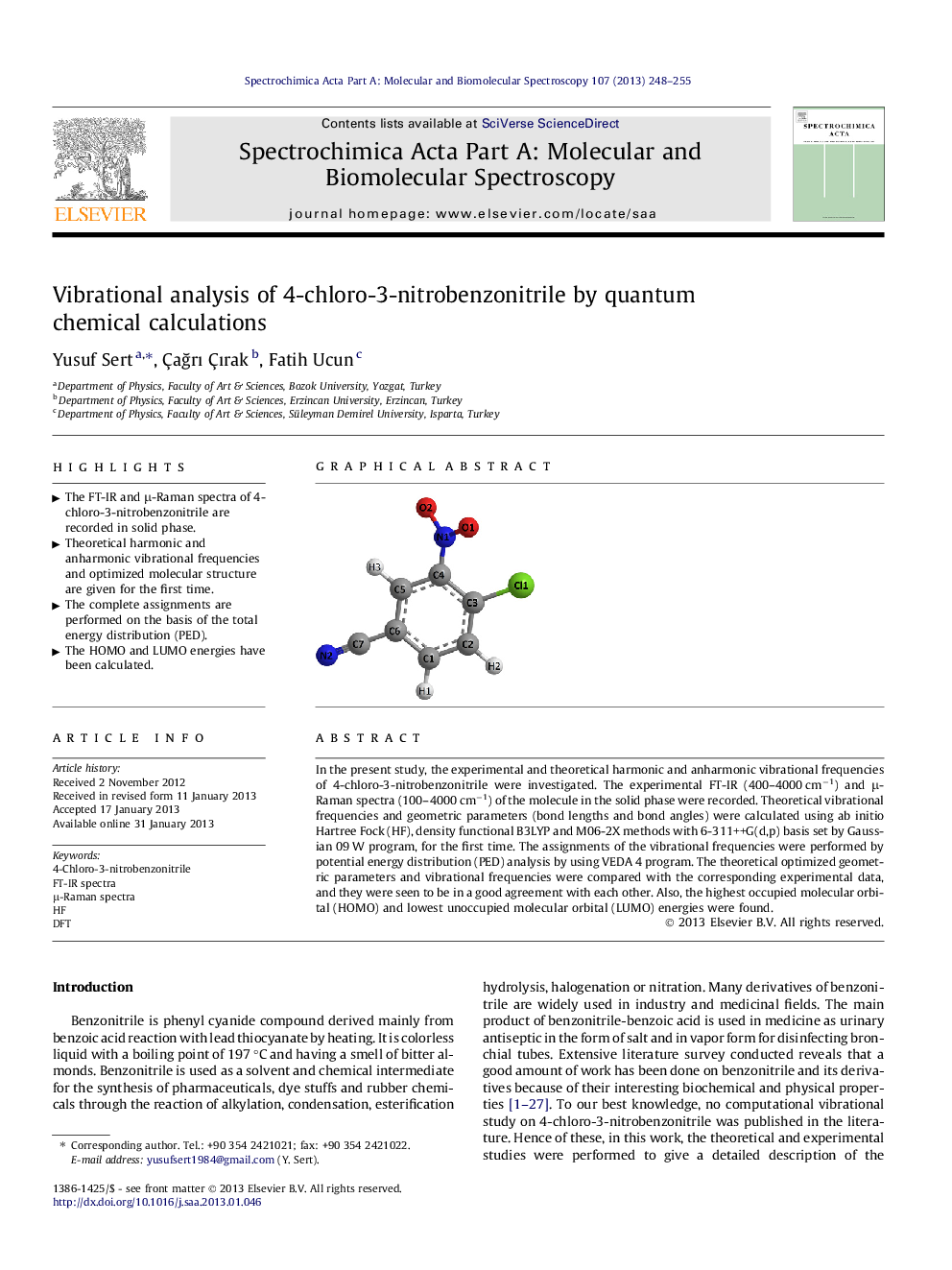
In the present study, the experimental and theoretical harmonic and anharmonic vibrational frequencies of 4-chloro-3-nitrobenzonitrile were investigated. The experimental FT-IR (400–4000 cm−1) and μ-Raman spectra (100–4000 cm−1) of the molecule in the solid phase were recorded. Theoretical vibrational frequencies and geometric parameters (bond lengths and bond angles) were calculated using ab initio Hartree Fock (HF), density functional B3LYP and M06-2X methods with 6-311++G(d,p) basis set by Gaussian 09 W program, for the first time. The assignments of the vibrational frequencies were performed by potential energy distribution (PED) analysis by using VEDA 4 program. The theoretical optimized geometric parameters and vibrational frequencies were compared with the corresponding experimental data, and they were seen to be in a good agreement with each other. Also, the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energies were found.
Figure optionsDownload as PowerPoint slideHighlights
► The FT-IR and μ-Raman spectra of 4-chloro-3-nitrobenzonitrile are recorded in solid phase.
► Theoretical harmonic and anharmonic vibrational frequencies and optimized molecular structure are given for the first time.
► The complete assignments are performed on the basis of the total energy distribution (PED).
► The HOMO and LUMO energies have been calculated.
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 107, 15 April 2013, Pages 248–255