| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1230991 | 1495258 | 2013 | 9 صفحه PDF | دانلود رایگان |
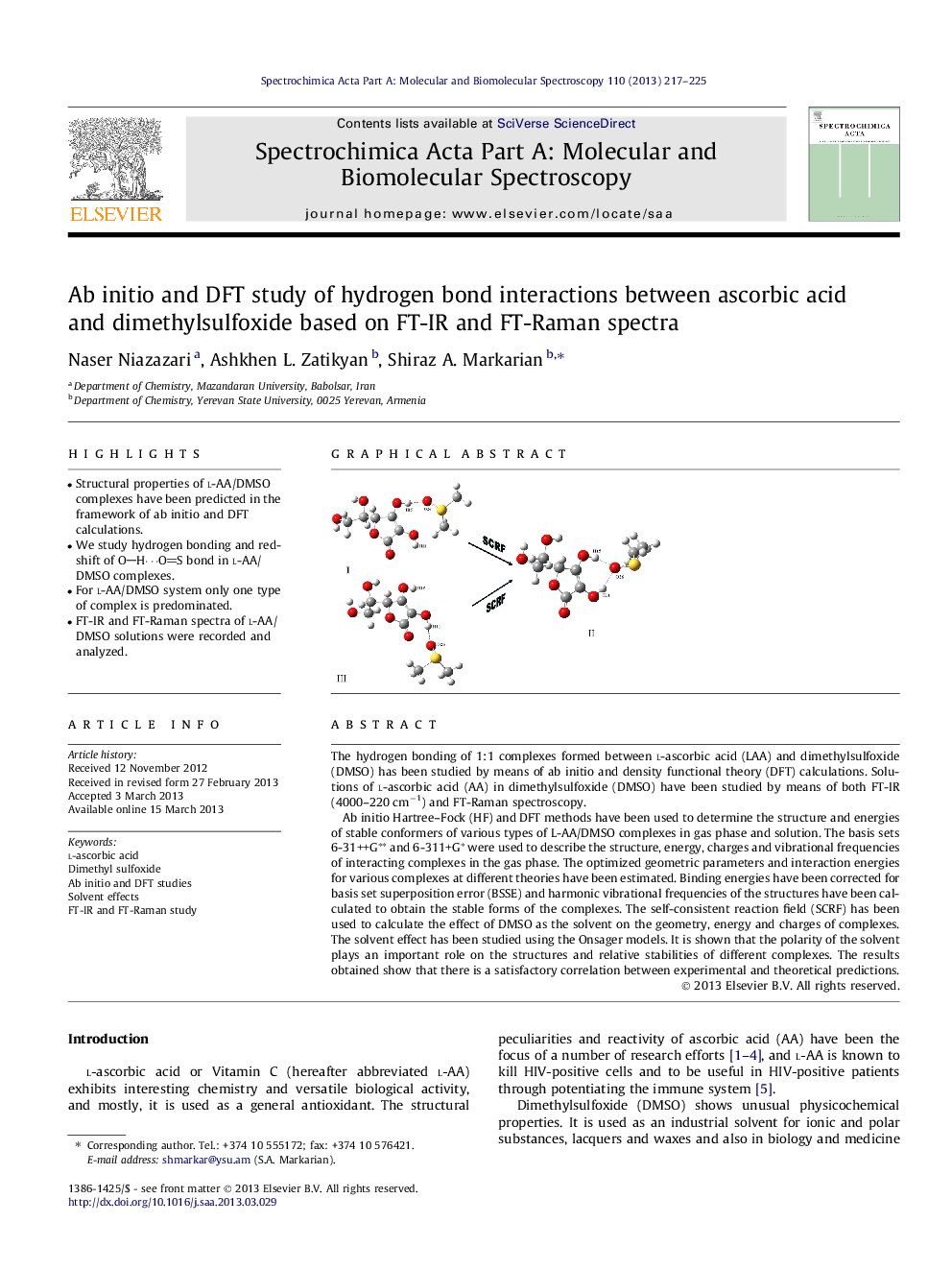
• Structural properties of l-AA/DMSO complexes have been predicted in the framework of ab initio and DFT calculations.
• We study hydrogen bonding and red-shift of OH⋯OS bond in l-AA/DMSO complexes.
• For l-AA/DMSO system only one type of complex is predominated.
• FT-IR and FT-Raman spectra of l-AA/DMSO solutions were recorded and analyzed.
The hydrogen bonding of 1:1 complexes formed between l-ascorbic acid (LAA) and dimethylsulfoxide (DMSO) has been studied by means of ab initio and density functional theory (DFT) calculations. Solutions of l-ascorbic acid (AA) in dimethylsulfoxide (DMSO) have been studied by means of both FT-IR (4000–220 cm−1) and FT-Raman spectroscopy.Ab initio Hartree–Fock (HF) and DFT methods have been used to determine the structure and energies of stable conformers of various types of L-AA/DMSO complexes in gas phase and solution. The basis sets 6-31++G∗∗ and 6-311+G∗ were used to describe the structure, energy, charges and vibrational frequencies of interacting complexes in the gas phase. The optimized geometric parameters and interaction energies for various complexes at different theories have been estimated. Binding energies have been corrected for basis set superposition error (BSSE) and harmonic vibrational frequencies of the structures have been calculated to obtain the stable forms of the complexes. The self-consistent reaction field (SCRF) has been used to calculate the effect of DMSO as the solvent on the geometry, energy and charges of complexes. The solvent effect has been studied using the Onsager models. It is shown that the polarity of the solvent plays an important role on the structures and relative stabilities of different complexes. The results obtained show that there is a satisfactory correlation between experimental and theoretical predictions.
Figure optionsDownload as PowerPoint slide
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 110, June 2013, Pages 217–225