| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1231002 | 1495258 | 2013 | 6 صفحه PDF | دانلود رایگان |
• Structural characterization of Fe(III)-1,2,4-triazole based complexes.
• All the complexes were characterized by spectroscopy techniques.
• All the complexes were excellent interpretation and discussion.
• All the complexes show an effective antibacterial activity.
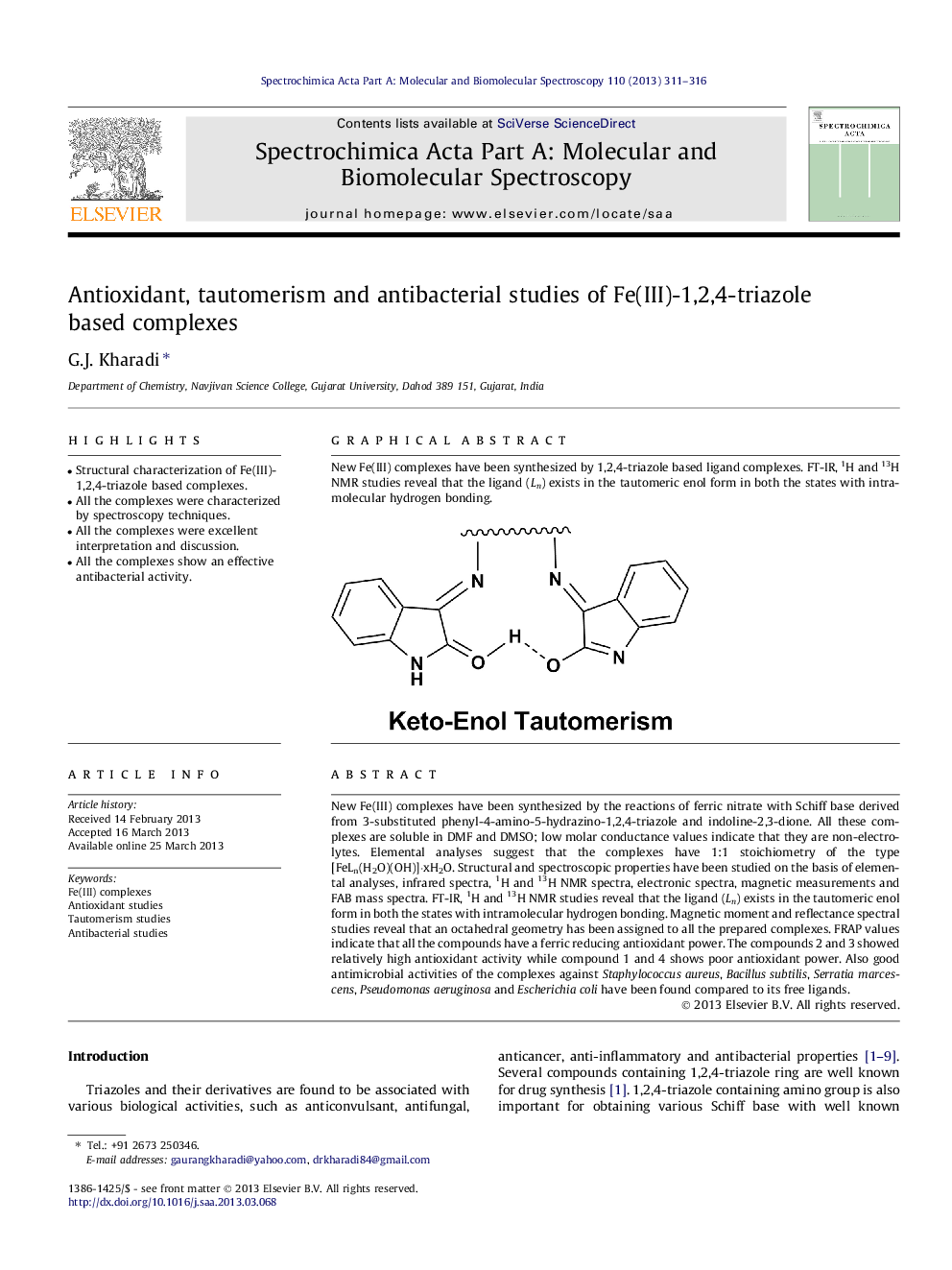
New Fe(III) complexes have been synthesized by the reactions of ferric nitrate with Schiff base derived from 3-substituted phenyl-4-amino-5-hydrazino-1,2,4-triazole and indoline-2,3-dione. All these complexes are soluble in DMF and DMSO; low molar conductance values indicate that they are non-electrolytes. Elemental analyses suggest that the complexes have 1:1 stoichiometry of the type [FeLn(H2O)(OH)]·xH2O. Structural and spectroscopic properties have been studied on the basis of elemental analyses, infrared spectra, 1H and 13H NMR spectra, electronic spectra, magnetic measurements and FAB mass spectra. FT-IR, 1H and 13H NMR studies reveal that the ligand (Ln) exists in the tautomeric enol form in both the states with intramolecular hydrogen bonding. Magnetic moment and reflectance spectral studies reveal that an octahedral geometry has been assigned to all the prepared complexes. FRAP values indicate that all the compounds have a ferric reducing antioxidant power. The compounds 2 and 3 showed relatively high antioxidant activity while compound 1 and 4 shows poor antioxidant power. Also good antimicrobial activities of the complexes against Staphylococcus aureus, Bacillus subtilis, Serratia marcescens, Pseudomonas aeruginosa and Escherichia coli have been found compared to its free ligands.
New Fe(III) complexes have been synthesized by 1,2,4-triazole based ligand complexes. FT-IR, 1H and 13H NMR studies reveal that the ligand (Ln) exists in the tautomeric enol form in both the states with intramolecular hydrogen bonding.Figure optionsDownload as PowerPoint slide
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 110, June 2013, Pages 311–316