| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1231242 | 1495264 | 2013 | 10 صفحه PDF | دانلود رایگان |
The FT-IR and FT-Raman vibrational spectra of ninhydrin have been recorded in the range 4000–400 cm−1and 3600–50 cm−1, respectively. A detailed vibrational spectral analysis has been carried out and assignments of the observed fundamental bands have been proposed on the basis of peak positions and relative intensities. The optimized molecular geometry, vibrational frequencies, atomic charges, dipole moment, rotational constants and several thermodynamic parameters in the ground state are calculated using ab initio HF and density functional B3LYP methods with 6-311++G(d,p) basis set combination. In order to find the most optimized geometry, the energy calculations are carried out for various possible conformers. Keto and enol forms of ninhydrin are also studied. The condensed summary of the principal NBOs shows the occupancy, orbital energy and the qualitative pattern of delocalization interactions of ninhydrin. The calculated HOMO–LUMO energies reveal that charge transfer occurs within the molecule. The predicted first hyperpolarizability also shows that the ninhydrin molecule have good optical quality and nonlinear optical (NLO) behavior. With the help of specific scaling procedures, the observed vibrational wave numbers in FT-IR and FT-Raman spectra are analyzed and assigned to different normal modes of the molecule.
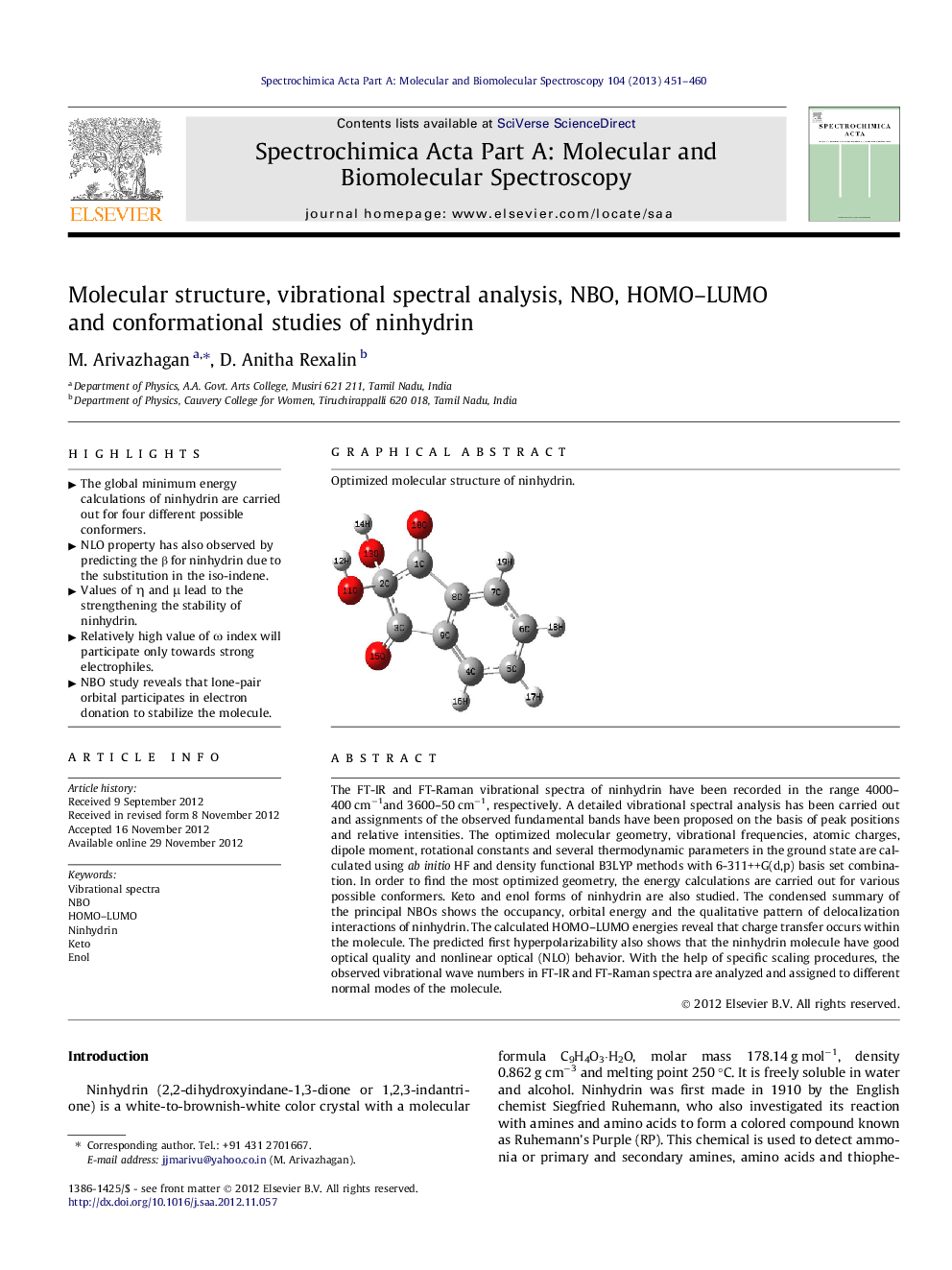
Optimized molecular structure of ninhydrin.Figure optionsDownload as PowerPoint slideHighlights
► The global minimum energy calculations of ninhydrin are carried out for four different possible conformers.
► NLO property has also observed by predicting the β for ninhydrin due to the substitution in the iso-indene.
► Values of η and μ lead to the strengthening the stability of ninhydrin.
► Relatively high value of ω index will participate only towards strong electrophiles.
► NBO study reveals that lone-pair orbital participates in electron donation to stabilize the molecule.
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 104, March 2013, Pages 451–460