| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1231268 | 1495254 | 2013 | 6 صفحه PDF | دانلود رایگان |
• The electrochemical redox process of molecules was spectrally resolved.
• The different interaction modes between molecule and substrate were spectrally distinguished.
• Relative Raman intensity and frequency shift are sensitive to interfacial interaction.
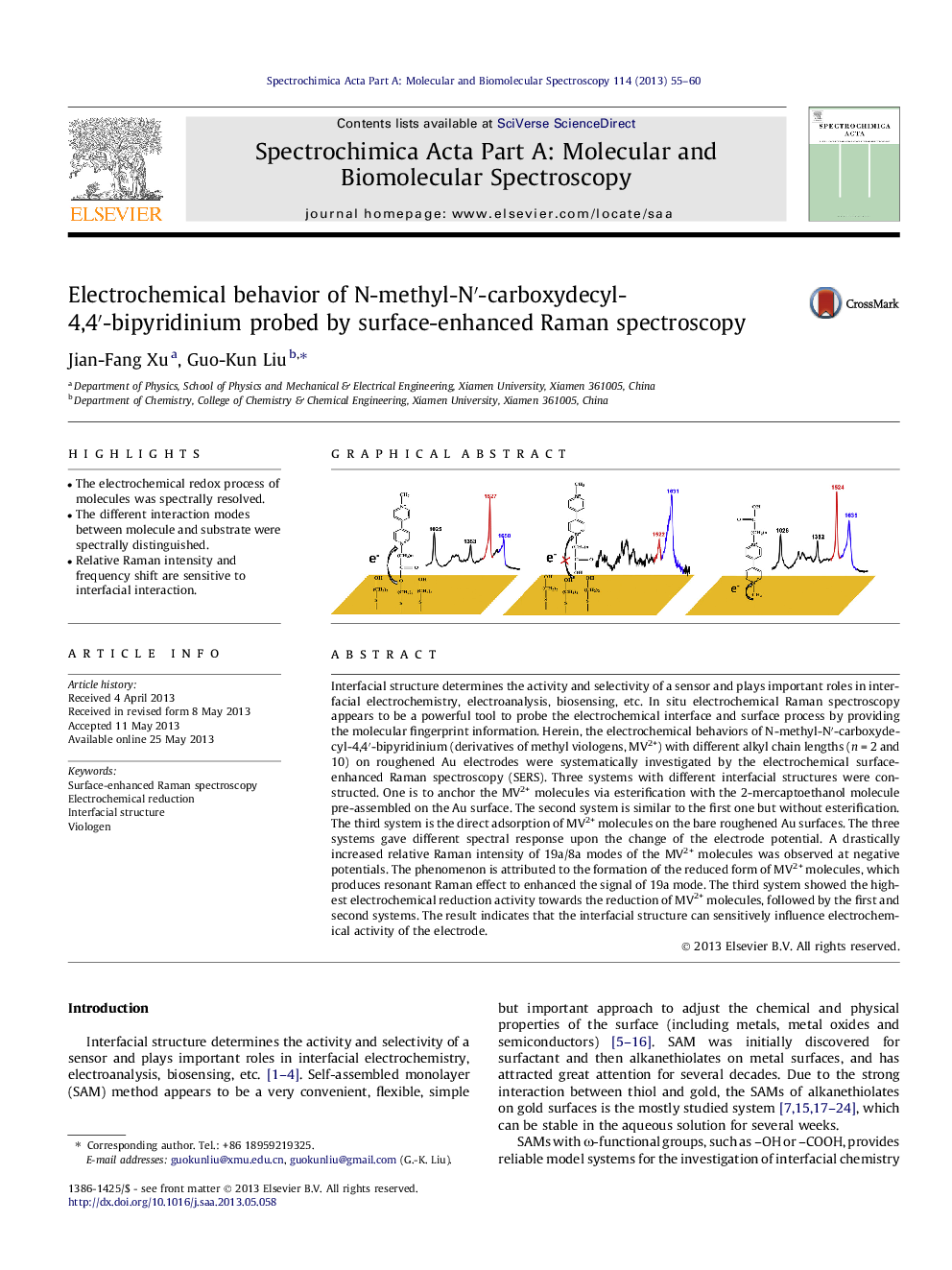
Interfacial structure determines the activity and selectivity of a sensor and plays important roles in interfacial electrochemistry, electroanalysis, biosensing, etc. In situ electrochemical Raman spectroscopy appears to be a powerful tool to probe the electrochemical interface and surface process by providing the molecular fingerprint information. Herein, the electrochemical behaviors of N-methyl-N′-carboxydecyl-4,4′-bipyridinium (derivatives of methyl viologens, MV2+) with different alkyl chain lengths (n = 2 and 10) on roughened Au electrodes were systematically investigated by the electrochemical surface-enhanced Raman spectroscopy (SERS). Three systems with different interfacial structures were constructed. One is to anchor the MV2+ molecules via esterification with the 2-mercaptoethanol molecule pre-assembled on the Au surface. The second system is similar to the first one but without esterification. The third system is the direct adsorption of MV2+ molecules on the bare roughened Au surfaces. The three systems gave different spectral response upon the change of the electrode potential. A drastically increased relative Raman intensity of 19a/8a modes of the MV2+ molecules was observed at negative potentials. The phenomenon is attributed to the formation of the reduced form of MV2+ molecules, which produces resonant Raman effect to enhanced the signal of 19a mode. The third system showed the highest electrochemical reduction activity towards the reduction of MV2+ molecules, followed by the first and second systems. The result indicates that the interfacial structure can sensitively influence electrochemical activity of the electrode.
Figure optionsDownload as PowerPoint slide
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 114, October 2013, Pages 55–60