| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1231624 | 1495219 | 2015 | 11 صفحه PDF | دانلود رایگان |
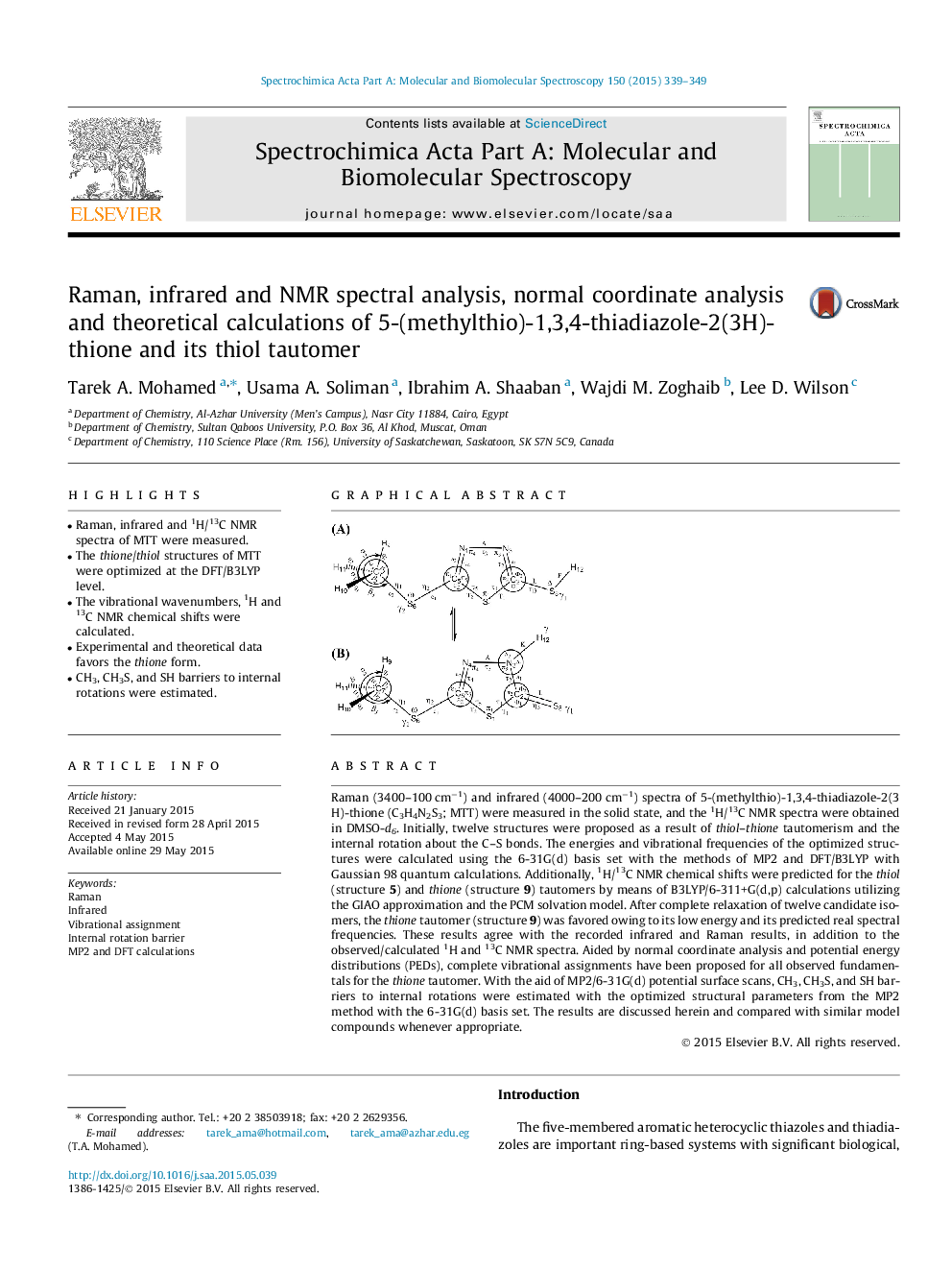
• Raman, infrared and 1H/13C NMR spectra of MTT were measured.
• The thione/thiol structures of MTT were optimized at the DFT/B3LYP level.
• The vibrational wavenumbers, 1H and 13C NMR chemical shifts were calculated.
• Experimental and theoretical data favors the thione form.
• CH3, CH3S, and SH barriers to internal rotations were estimated.
Raman (3400–100 cm−1) and infrared (4000–200 cm−1) spectra of 5-(methylthio)-1,3,4-thiadiazole-2(3H)-thione (C3H4N2S3; MTT) were measured in the solid state, and the 1H/13C NMR spectra were obtained in DMSO-d6. Initially, twelve structures were proposed as a result of thiol–thione tautomerism and the internal rotation about the C–S bonds. The energies and vibrational frequencies of the optimized structures were calculated using the 6-31G(d) basis set with the methods of MP2 and DFT/B3LYP with Gaussian 98 quantum calculations. Additionally, 1H/13C NMR chemical shifts were predicted for the thiol (structure 5) and thione (structure 9) tautomers by means of B3LYP/6-311+G(d,p) calculations utilizing the GIAO approximation and the PCM solvation model. After complete relaxation of twelve candidate isomers, the thione tautomer (structure 9) was favored owing to its low energy and its predicted real spectral frequencies. These results agree with the recorded infrared and Raman results, in addition to the observed/calculated 1H and 13C NMR spectra. Aided by normal coordinate analysis and potential energy distributions (PEDs), complete vibrational assignments have been proposed for all observed fundamentals for the thione tautomer. With the aid of MP2/6-31G(d) potential surface scans, CH3, CH3S, and SH barriers to internal rotations were estimated with the optimized structural parameters from the MP2 method with the 6-31G(d) basis set. The results are discussed herein and compared with similar model compounds whenever appropriate.
Figure optionsDownload as PowerPoint slide
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 150, 5 November 2015, Pages 339–349