| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1231861 | 1495263 | 2013 | 6 صفحه PDF | دانلود رایگان |
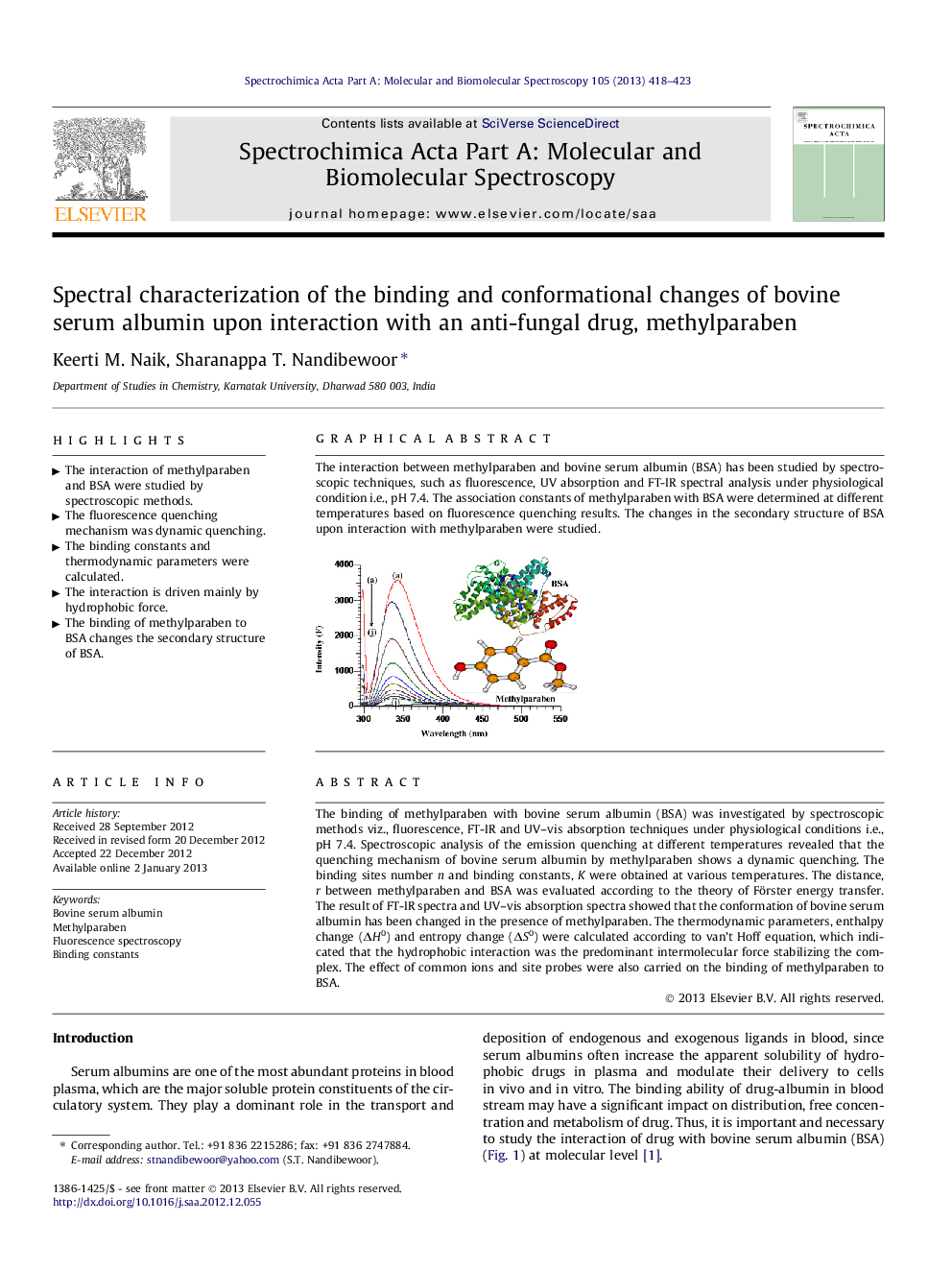
The binding of methylparaben with bovine serum albumin (BSA) was investigated by spectroscopic methods viz., fluorescence, FT-IR and UV–vis absorption techniques under physiological conditions i.e., pH 7.4. Spectroscopic analysis of the emission quenching at different temperatures revealed that the quenching mechanism of bovine serum albumin by methylparaben shows a dynamic quenching. The binding sites number n and binding constants, K were obtained at various temperatures. The distance, r between methylparaben and BSA was evaluated according to the theory of Förster energy transfer. The result of FT-IR spectra and UV–vis absorption spectra showed that the conformation of bovine serum albumin has been changed in the presence of methylparaben. The thermodynamic parameters, enthalpy change (ΔH0) and entropy change (ΔS0) were calculated according to van’t Hoff equation, which indicated that the hydrophobic interaction was the predominant intermolecular force stabilizing the complex. The effect of common ions and site probes were also carried on the binding of methylparaben to BSA.
The interaction between methylparaben and bovine serum albumin (BSA) has been studied by spectroscopic techniques, such as fluorescence, UV absorption and FT-IR spectral analysis under physiological condition i.e., pH 7.4. The association constants of methylparaben with BSA were determined at different temperatures based on fluorescence quenching results. The changes in the secondary structure of BSA upon interaction with methylparaben were studied.Figure optionsDownload as PowerPoint slideHighlights
► The interaction of methylparaben and BSA were studied by spectroscopic methods.
► The fluorescence quenching mechanism was dynamic quenching.
► The binding constants and thermodynamic parameters were calculated.
► The interaction is driven mainly by hydrophobic force.
► The binding of methylparaben to BSA changes the secondary structure of BSA.
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 105, 15 March 2013, Pages 418–423