| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1231920 | 1495223 | 2015 | 9 صفحه PDF | دانلود رایگان |
• A charge transfer complex has been prepared and confirmed by spectral investigations.
• Spectrophotometric studies are made in different polar solvents.
• Benesi–Hildebrand equation has been used to determine KCT, εCT, and ECT of the CT complex.
• Thermal analysis (TGA–DTA) has been performed to confirm the thermal fragmentation and the stability of the CT complex.
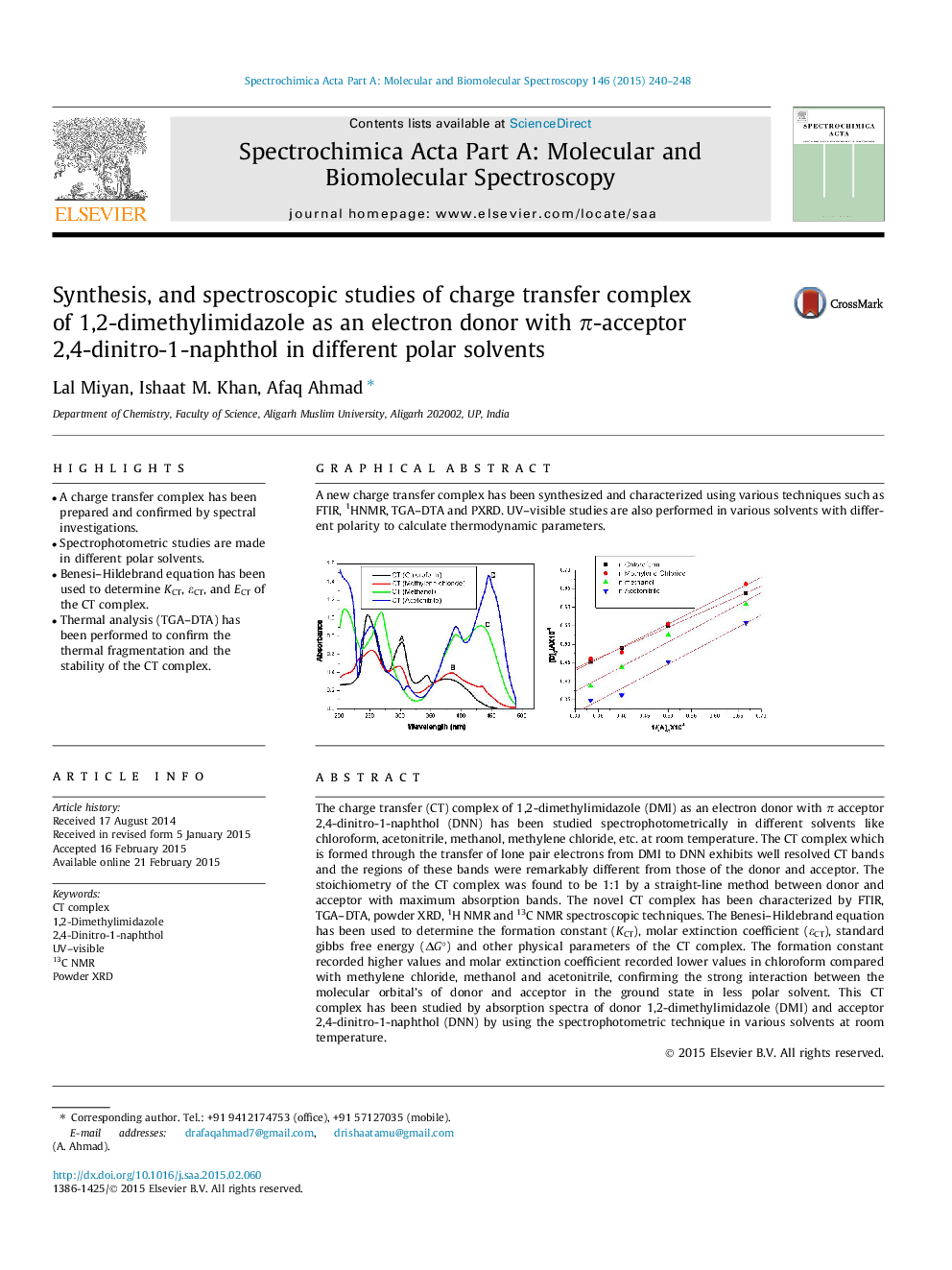
The charge transfer (CT) complex of 1,2-dimethylimidazole (DMI) as an electron donor with π acceptor 2,4-dinitro-1-naphthol (DNN) has been studied spectrophotometrically in different solvents like chloroform, acetonitrile, methanol, methylene chloride, etc. at room temperature. The CT complex which is formed through the transfer of lone pair electrons from DMI to DNN exhibits well resolved CT bands and the regions of these bands were remarkably different from those of the donor and acceptor. The stoichiometry of the CT complex was found to be 1:1 by a straight-line method between donor and acceptor with maximum absorption bands. The novel CT complex has been characterized by FTIR, TGA–DTA, powder XRD, 1H NMR and 13C NMR spectroscopic techniques. The Benesi–Hildebrand equation has been used to determine the formation constant (KCT), molar extinction coefficient (εCT), standard gibbs free energy (ΔG°) and other physical parameters of the CT complex. The formation constant recorded higher values and molar extinction coefficient recorded lower values in chloroform compared with methylene chloride, methanol and acetonitrile, confirming the strong interaction between the molecular orbital’s of donor and acceptor in the ground state in less polar solvent. This CT complex has been studied by absorption spectra of donor 1,2-dimethylimidazole (DMI) and acceptor 2,4-dinitro-1-naphthol (DNN) by using the spectrophotometric technique in various solvents at room temperature.
A new charge transfer complex has been synthesized and characterized using various techniques such as FTIR, 1HNMR, TGA–DTA and PXRD. UV–visible studies are also performed in various solvents with different polarity to calculate thermodynamic parameters.Figure optionsDownload as PowerPoint slide
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 146, 5 July 2015, Pages 240–248