| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1231989 | 1495278 | 2012 | 5 صفحه PDF | دانلود رایگان |
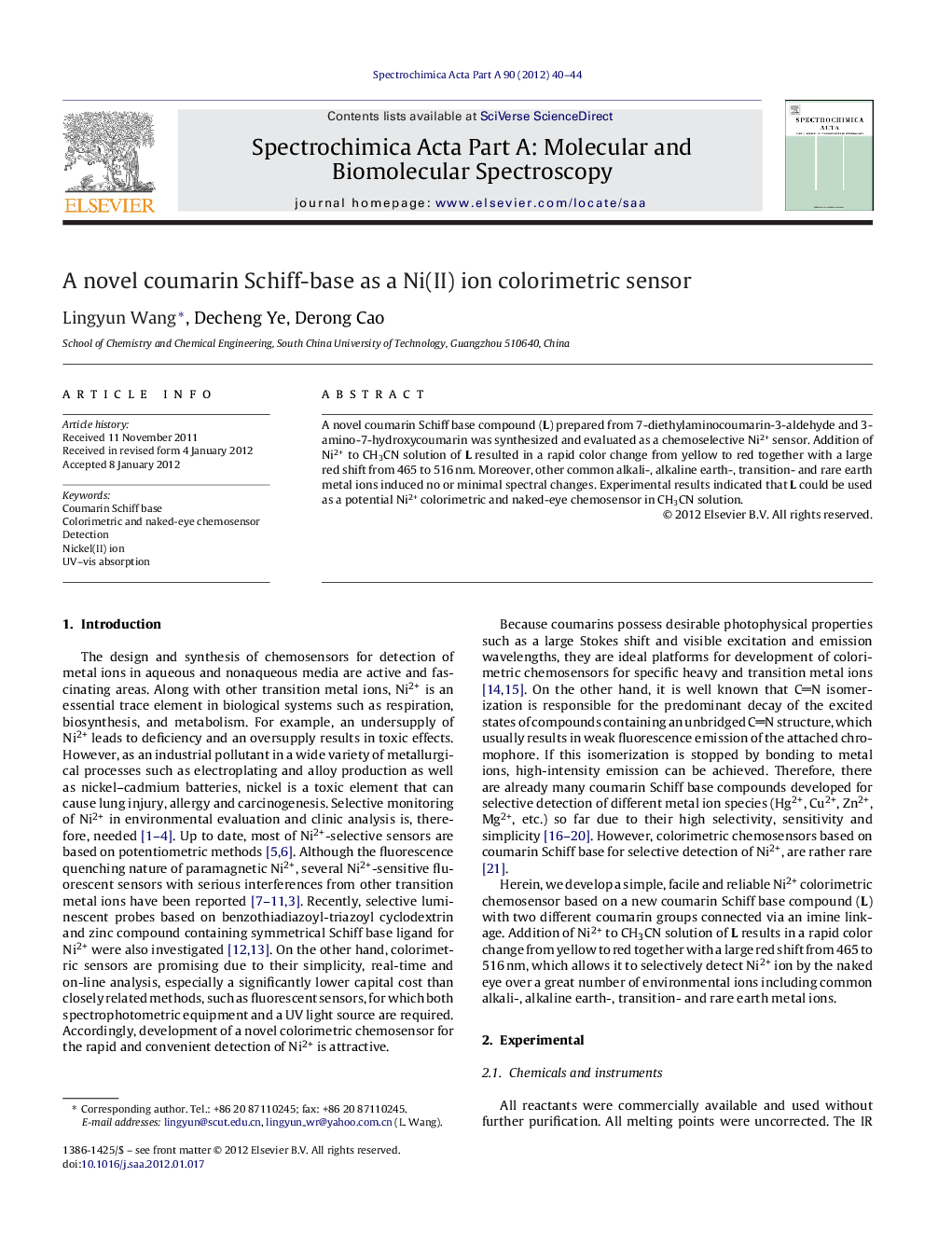
A novel coumarin Schiff base compound (L) prepared from 7-diethylaminocoumarin-3-aldehyde and 3-amino-7-hydroxycoumarin was synthesized and evaluated as a chemoselective Ni2+ sensor. Addition of Ni2+ to CH3CN solution of L resulted in a rapid color change from yellow to red together with a large red shift from 465 to 516 nm. Moreover, other common alkali-, alkaline earth-, transition- and rare earth metal ions induced no or minimal spectral changes. Experimental results indicated that L could be used as a potential Ni2+ colorimetric and naked-eye chemosensor in CH3CN solution.
Figure photographs of L color changes (10.0 μM) with gradual addition of Ni2+ from left to right: 0, 4, 10, 20 and 50 μM, respectively in CH3CN solution. A novel coumarin Schiff base compound (L) prepared from 7-diethylaminocoumarin-3-aldehyde and 3-amino-7-hydroxycoumarin was evaluated as a chemoselective Ni2+ sensor. Addition of Ni2+ to CH3CN solution of L resulted in a rapid color change from yellow to red together with a large red shift from 465 to 516 nm, indicating that L could be used as a potential Ni2+ colorimetric and naked-eye chemosensor in CH3CN solution.Figure optionsDownload as PowerPoint slideHighlights
► A novel coumarin Schiff base compound (L) prepared from 7-diethylaminocoumarin-3-aldehyde and 3-amino-7-hydroxycoumarin was synthesized.
► Colorimetric chemosensor (yellow to red) based on coumarin Schiff base for selective detection of Ni2+ was reported.
► Compound L possesses a good selectivity toward Ni2+ over other competitive cations (Na+, K+, Ca2+, Mg2+, Ba2+, Hg2+, Cu2+, Zn2+, Cd2+, Sb3+ and Fe3+).
► Absorption spectrum and fluorescence emission spectrum were used to study selective recognition of Ni2+ with L.
► The association constant of L with Ni2+ in CH3CN solution was calculated to be 2.9 × 104 M−1.
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 90, May 2012, Pages 40–44