| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1232064 | 1495265 | 2013 | 7 صفحه PDF | دانلود رایگان |
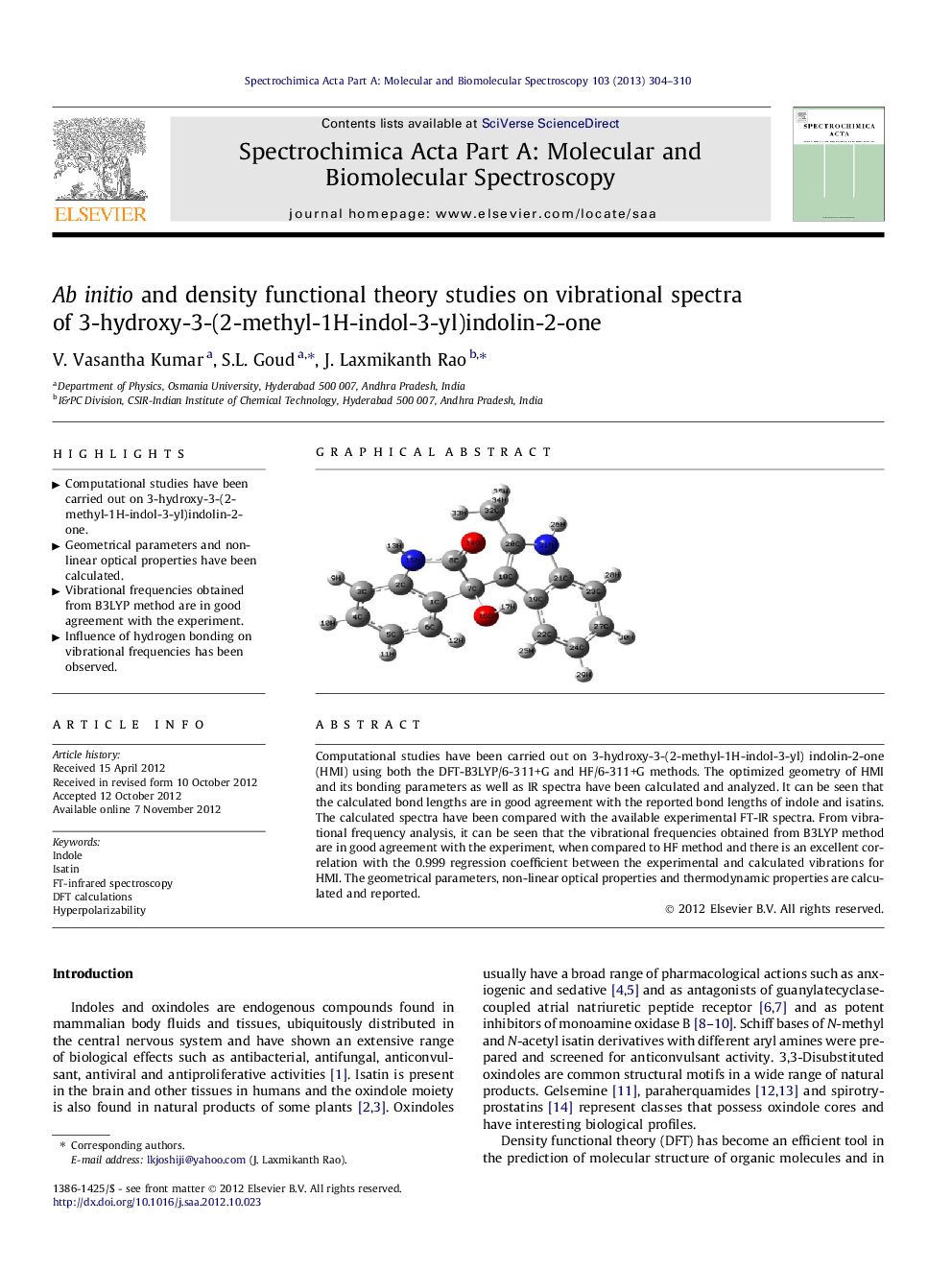
Computational studies have been carried out on 3-hydroxy-3-(2-methyl-1H-indol-3-yl) indolin-2-one (HMI) using both the DFT-B3LYP/6-311+G and HF/6-311+G methods. The optimized geometry of HMI and its bonding parameters as well as IR spectra have been calculated and analyzed. It can be seen that the calculated bond lengths are in good agreement with the reported bond lengths of indole and isatins. The calculated spectra have been compared with the available experimental FT-IR spectra. From vibrational frequency analysis, it can be seen that the vibrational frequencies obtained from B3LYP method are in good agreement with the experiment, when compared to HF method and there is an excellent correlation with the 0.999 regression coefficient between the experimental and calculated vibrations for HMI. The geometrical parameters, non-linear optical properties and thermodynamic properties are calculated and reported.
Figure optionsDownload as PowerPoint slideHighlights
► Computational studies have been carried out on 3-hydroxy-3-(2-methyl-1H-indol-3-yl)indolin-2-one.
► Geometrical parameters and non-linear optical properties have been calculated.
► Vibrational frequencies obtained from B3LYP method are in good agreement with the experiment.
► Influence of hydrogen bonding on vibrational frequencies has been observed.
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 103, 15 February 2013, Pages 304–310