| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1232288 | 1495271 | 2012 | 5 صفحه PDF | دانلود رایگان |
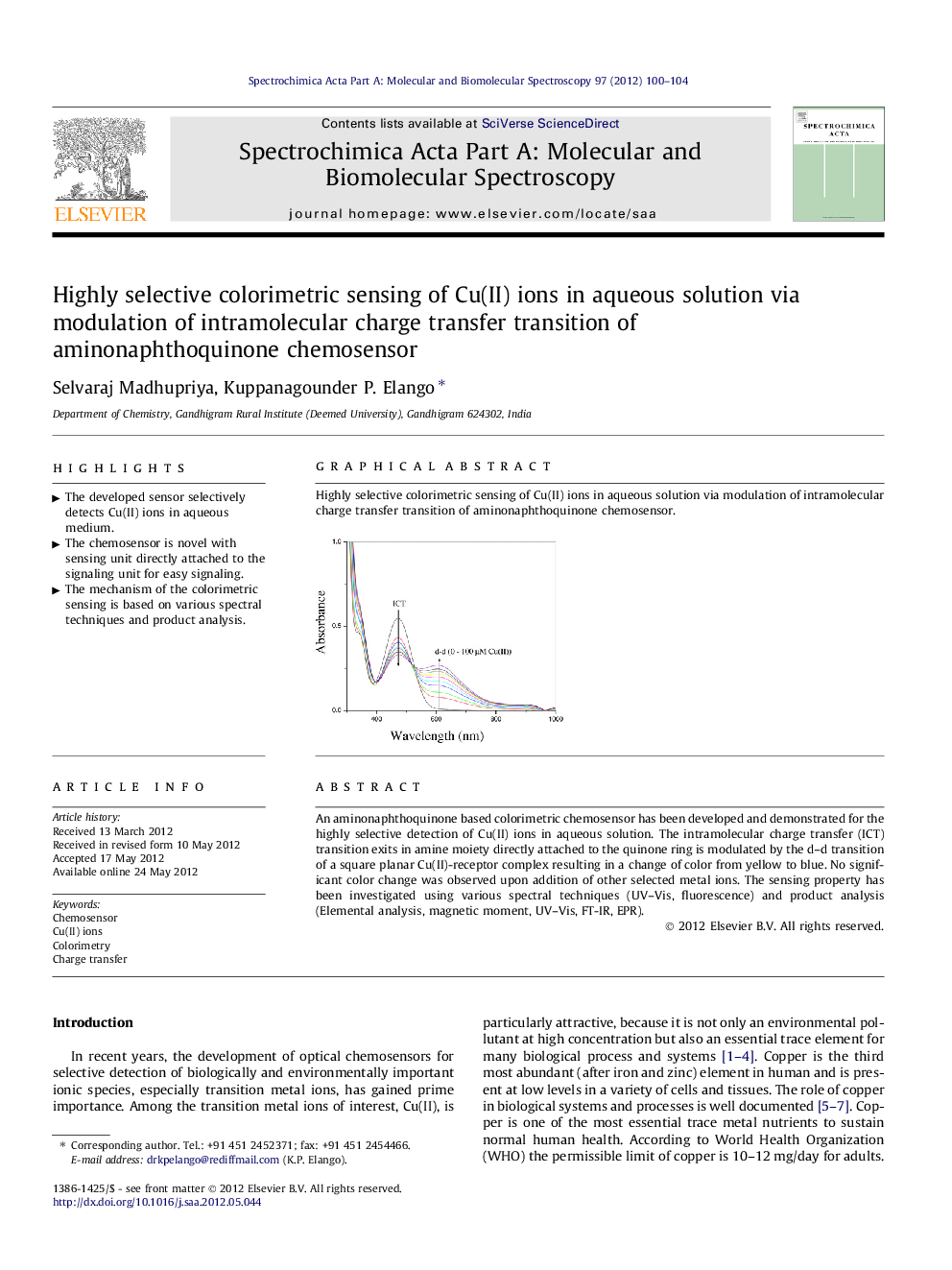
An aminonaphthoquinone based colorimetric chemosensor has been developed and demonstrated for the highly selective detection of Cu(II) ions in aqueous solution. The intramolecular charge transfer (ICT) transition exits in amine moiety directly attached to the quinone ring is modulated by the d–d transition of a square planar Cu(II)-receptor complex resulting in a change of color from yellow to blue. No significant color change was observed upon addition of other selected metal ions. The sensing property has been investigated using various spectral techniques (UV–Vis, fluorescence) and product analysis (Elemental analysis, magnetic moment, UV–Vis, FT-IR, EPR).
Highly selective colorimetric sensing of Cu(II) ions in aqueous solution via modulation of intramolecular charge transfer transition of aminonaphthoquinone chemosensor.Figure optionsDownload as PowerPoint slideHighlights
► The developed sensor selectively detects Cu(II) ions in aqueous medium.
► The chemosensor is novel with sensing unit directly attached to the signaling unit for easy signaling.
► The mechanism of the colorimetric sensing is based on various spectral techniques and product analysis.
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 97, November 2012, Pages 100–104