| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1232309 | 1495271 | 2012 | 11 صفحه PDF | دانلود رایگان |
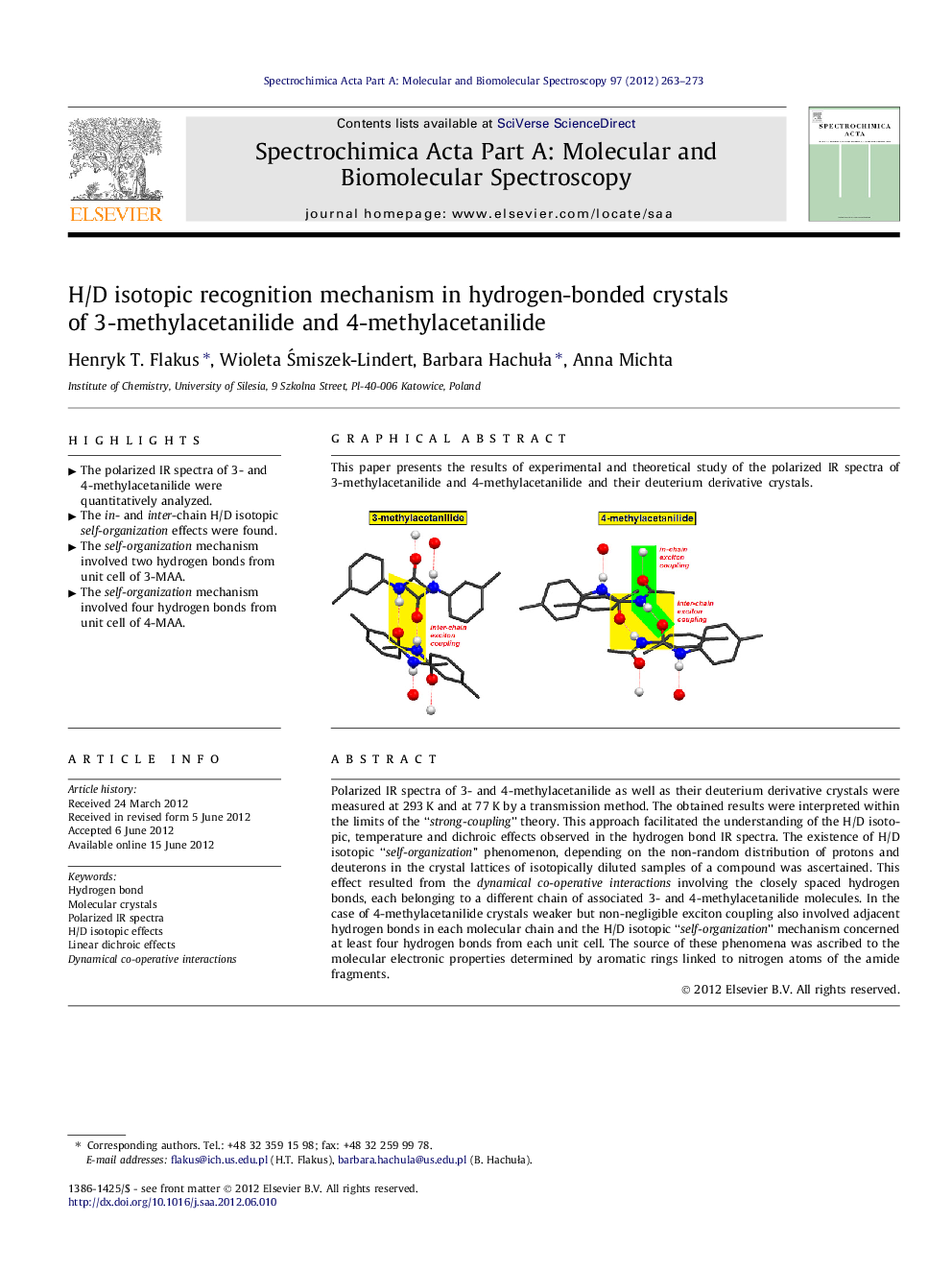
Polarized IR spectra of 3- and 4-methylacetanilide as well as their deuterium derivative crystals were measured at 293 K and at 77 K by a transmission method. The obtained results were interpreted within the limits of the “strong-coupling” theory. This approach facilitated the understanding of the H/D isotopic, temperature and dichroic effects observed in the hydrogen bond IR spectra. The existence of H/D isotopic “self-organization” phenomenon, depending on the non-random distribution of protons and deuterons in the crystal lattices of isotopically diluted samples of a compound was ascertained. This effect resulted from the dynamical co-operative interactions involving the closely spaced hydrogen bonds, each belonging to a different chain of associated 3- and 4-methylacetanilide molecules. In the case of 4-methylacetanilide crystals weaker but non-negligible exciton coupling also involved adjacent hydrogen bonds in each molecular chain and the H/D isotopic “self-organization” mechanism concerned at least four hydrogen bonds from each unit cell. The source of these phenomena was ascribed to the molecular electronic properties determined by aromatic rings linked to nitrogen atoms of the amide fragments.
This paper presents the results of experimental and theoretical study of the polarized IR spectra of 3-methylacetanilide and 4-methylacetanilide and their deuterium derivative crystals.Figure optionsDownload as PowerPoint slideHighlights
► The polarized IR spectra of 3- and 4-methylacetanilide were quantitatively analyzed.
► The in- and inter-chain H/D isotopic self-organization effects were found.
► The self-organization mechanism involved two hydrogen bonds from unit cell of 3-MAA.
► The self-organization mechanism involved four hydrogen bonds from unit cell of 4-MAA.
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 97, November 2012, Pages 263–273