| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1232352 | 1495271 | 2012 | 6 صفحه PDF | دانلود رایگان |
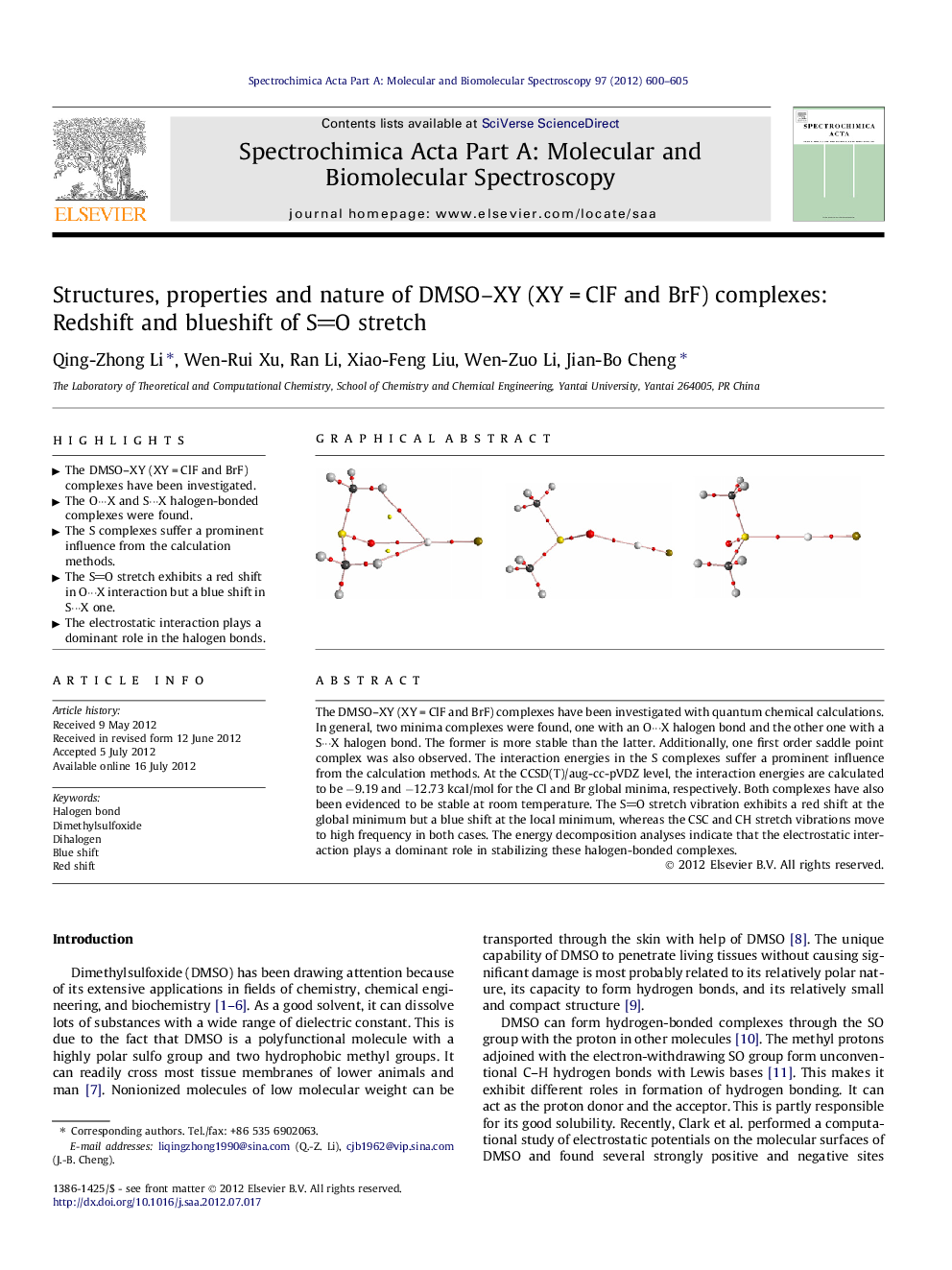
The DMSO–XY (XY = ClF and BrF) complexes have been investigated with quantum chemical calculations. In general, two minima complexes were found, one with an O···X halogen bond and the other one with a S···X halogen bond. The former is more stable than the latter. Additionally, one first order saddle point complex was also observed. The interaction energies in the S complexes suffer a prominent influence from the calculation methods. At the CCSD(T)/aug-cc-pVDZ level, the interaction energies are calculated to be −9.19 and −12.73 kcal/mol for the Cl and Br global minima, respectively. Both complexes have also been evidenced to be stable at room temperature. The SO stretch vibration exhibits a red shift at the global minimum but a blue shift at the local minimum, whereas the CSC and CH stretch vibrations move to high frequency in both cases. The energy decomposition analyses indicate that the electrostatic interaction plays a dominant role in stabilizing these halogen-bonded complexes.
Figure optionsDownload as PowerPoint slideHighlights
► The DMSO–XY (XY = ClF and BrF) complexes have been investigated.
► The O···X and S···X halogen-bonded complexes were found.
► The S complexes suffer a prominent influence from the calculation methods.
► The SO stretch exhibits a red shift in O···X interaction but a blue shift in S···X one.
► The electrostatic interaction plays a dominant role in the halogen bonds.
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 97, November 2012, Pages 600–605