| کد مقاله | کد نشریه | سال انتشار | مقاله انگلیسی | نسخه تمام متن |
|---|---|---|---|---|
| 1232406 | 1495228 | 2015 | 5 صفحه PDF | دانلود رایگان |
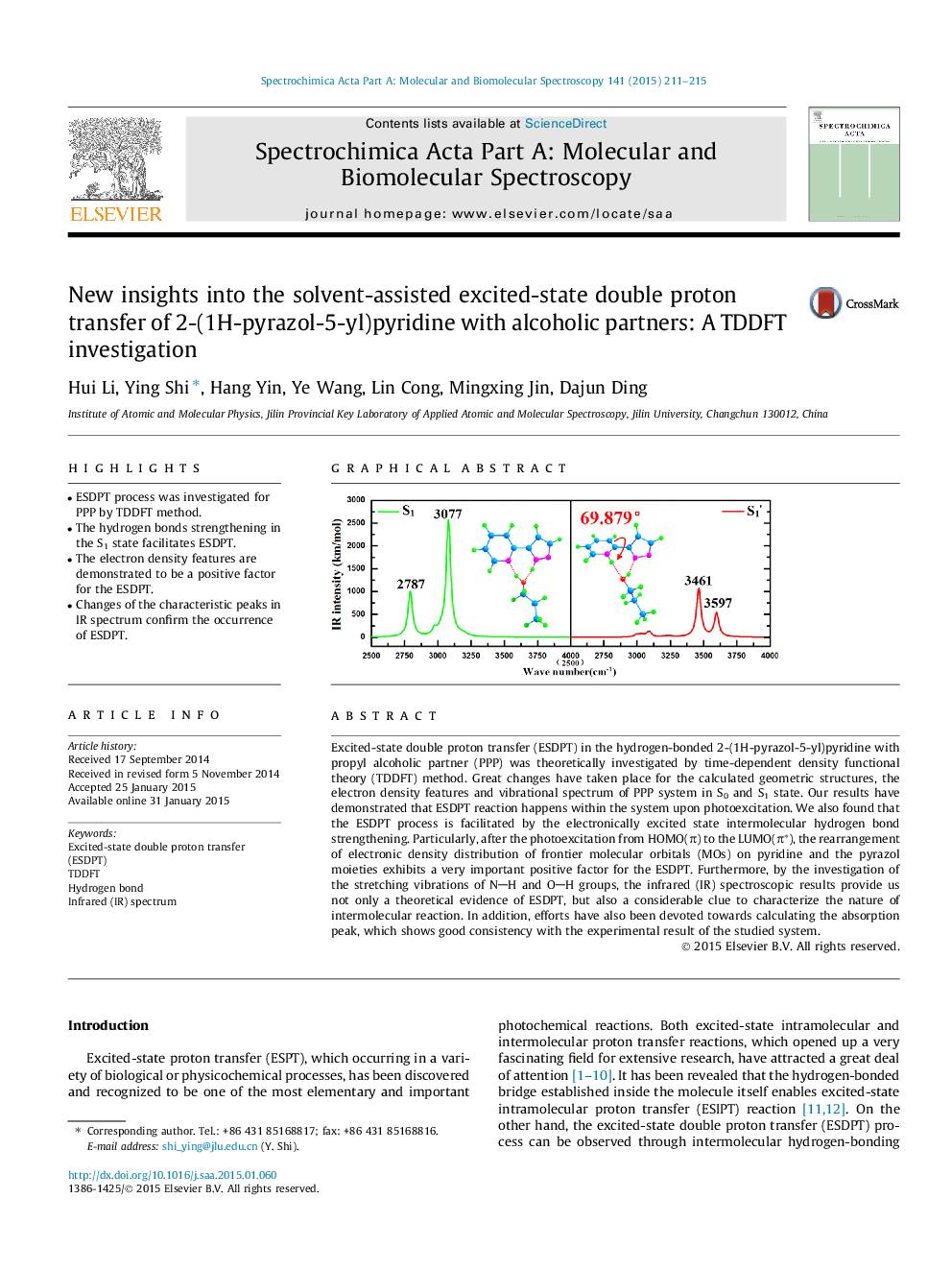
• ESDPT process was investigated for PPP by TDDFT method.
• The hydrogen bonds strengthening in the S1 state facilitates ESDPT.
• The electron density features are demonstrated to be a positive factor for the ESDPT.
• Changes of the characteristic peaks in IR spectrum confirm the occurrence of ESDPT.
Excited-state double proton transfer (ESDPT) in the hydrogen-bonded 2-(1H-pyrazol-5-yl)pyridine with propyl alcoholic partner (PPP) was theoretically investigated by time-dependent density functional theory (TDDFT) method. Great changes have taken place for the calculated geometric structures, the electron density features and vibrational spectrum of PPP system in S0 and S1 state. Our results have demonstrated that ESDPT reaction happens within the system upon photoexcitation. We also found that the ESDPT process is facilitated by the electronically excited state intermolecular hydrogen bond strengthening. Particularly, after the photoexcitation from HOMO(π) to the LUMO(π∗), the rearrangement of electronic density distribution of frontier molecular orbitals (MOs) on pyridine and the pyrazol moieties exhibits a very important positive factor for the ESDPT. Furthermore, by the investigation of the stretching vibrations of NH and OH groups, the infrared (IR) spectroscopic results provide us not only a theoretical evidence of ESDPT, but also a considerable clue to characterize the nature of intermolecular reaction. In addition, efforts have also been devoted towards calculating the absorption peak, which shows good consistency with the experimental result of the studied system.
Figure optionsDownload as PowerPoint slide
Journal: Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy - Volume 141, 15 April 2015, Pages 211–215